While pacemaker have been instrumental in the treatment of many patients with heart rhythm disorders, their bulky design and reliance on leads can limit their usefulness and pose a risk of heart damage or infection. Researchers at the University of Chicago have cut the wires, reduced the size and expanded the capabilities of current designs.
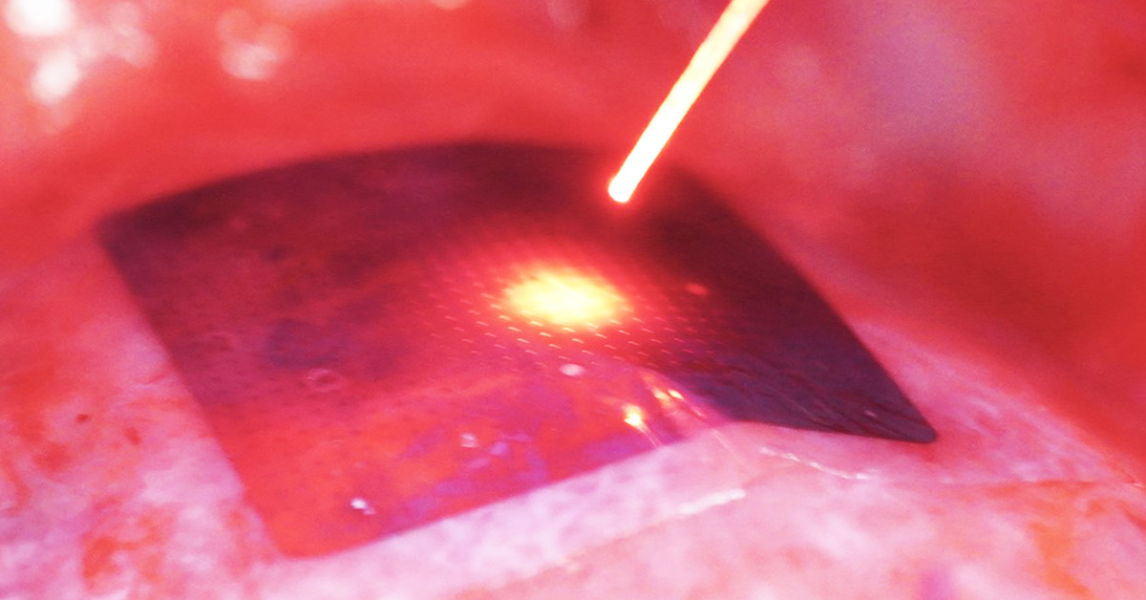
Its new technology is a flexible, lightweight silicone membrane that adapts to the surface of the heart and delivers electrical current when and where light rays hit the device. in a recent study in NatureThe authors used their strategy to regulate cardiac activity in a variety of living animals. The results suggest that the system could one day provide a high degree of control over irregular heart activity, while posing less risk and burden to patients than traditional pacemakers.
The rhythmic movements of the heart are the result of electrical signals that propagate through tissue, causing the different chambers of the heart to contract in a particular order. Cardiovascular disease or acute events, such as heart attacks, that damage heart tissue can slow or alter this life-sustaining rhythm.
Current treatments typically involve the use of pacemakers that apply electrical stimulation to restore or correct cardiac activity. These devices typically send impulses through wires or wires surgically housed in the chambers of the heart.
“With traditional lead-based pacemakers, pacing is limited to the site where the leads are implanted. But this is not ideal because sometimes the optimal location for pacing changes over time,” said co-corresponding author Bozhi Tian, Ph.D., professor of chemistry at the University of Chicago. “Basically, with our method, you have more freedom to adjust the location of the stimulation.”
This freedom arises from a process called the photovoltaic effect, in which light rays cause a material to produce an electric current. The new pacemaker technology mirrors the structure of a solar cell (its two layers of silicon prepared to facilitate electrical flow), but instead of storing the electrical charge, the device distributes it over the underlying heart muscle.
In their study, Tian and his colleagues first evaluated their pacemaker device on cardiac cell samples and ex vivo animal hearts. By shining a laser on the membrane, they managed to cause contractions in both models.
They then tested their method on live mice, rats and pigs. After applying the device, the team stimulated heart tissue in multiple locations, sometimes simultaneously. This coordinated stimulation is essential for a heart failure treatment called cardiac resynchronization therapy (CRT). With CRT, doctors use pacemakers to restore and coordinate activity in the upper and lower chambers of the heart.
“Currently available pacemakers are capable of pacing only one or two sites and often fail to achieve synchronicity for approximately a third of patients receiving CRT,” said co-author Narutoshi Hibino, M.D., Ph.D., professor of surgery at the University of Chicago Medical Center. “Multi-site stimulation addresses this limitation by synchronizing movement in different areas of the heart.”
Throughout all experiments, the authors managed to stimulate hearts to beat steadily over a wide range of target frequencies using healthy animals. In an experiment they obtained similar results in an injured heart model. The outcome of these tests was promising, but limited by the fact that they used a highly invasive approach to place the device in the heart and stimulate it with a light source.
The team devised a less intrusive procedure for their final test, performed on a live pig. They inserted a collapsed version of their device through a small incision to place it in the heart. The researchers then slid an optical fiber through the same opening to illuminate the device and regulate the animal’s heart rate.
While the study showed that the new approach is valid for short-term procedures such as CRT, the authors believe their device could find use in long-term applications. For the treatment of chronic diseases, a small wireless LED implanted under a patient’s skin could remotely trigger stimulation, Tian said.
The authors intend to conduct experiments on animals over longer periods of time to strengthen the arguments in favor of their technique. Additionally, they plan to integrate their technology with sensors attached to the skin to measure cardiac activity, a capability necessary for the system to provide stimulation at the right time.
“The authors have not only developed a new way to perform cardiac electrical stimulation, but have also carefully considered how this method could be translated into the clinic. It will be interesting to see how this technology evolves,” said Jessica Falcone, Ph.D., director of the Medical Devices Program at the National Institute of Biomedical Imaging and Bioengineering (NIBIB).
This research was partially funded by a NIBIB grant (R56EB034289).
This prominent scientist describes a basic research finding. Basic research increases our understanding of human behavior and biology, which is critical to promoting new and better ways to prevent, diagnose, and treat diseases. Science is an unpredictable and incremental process: each research advance builds on past discoveries, often in unexpected ways. Most clinical advances would not be possible without knowledge of fundamental basic research.
Study reference: Pengju Li et al. Monolithic silicon for high spatiotemporal translational photostimulation. Nature. DOI: 10.1038/s41586-024-07016-9