Neurological disorders such as Parkinson’s disease and epilepsy have had some success in treating with deep brain stimulation devices, but require invasive and expensive surgical implantation. Now, engineers funded by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) have devised a system to control motor activity in the brain without the implantation of a surgical device, a first step toward non-invasive cell-specific brain stimulation therapies.
The research team, led by Hong Chen, PhD, associate professor of biomedical engineering at the McKelvey School of Engineering and of radiation oncology at Washington University School of Medicine in St. Louis, has combined sound, heat and genetics to create a non-invasive system capable of stimulating specific cell types deep in the brain.
They have called this approach sonothermogenetics.
“Sono” refers to the ultrasound part of the technology. It takes advantage of the ability of focused ultrasound to penetrate deep into the brain, but it has an effect on only a small area of the brain cells it targets. “Thermo” refers to a small amount of heat that is created when the ultrasound reaches its target. “Genetics” refers to the fact that the equipment uses genes that code for proteins that can be turned on and off by the small amount of heat generated by focused ultrasound.
“This engineering approach could help address the public health problem created by the growing number of people affected by neurological disorders worldwide by offering non-invasive treatment alternatives to patients,” said Randy King, PhD, NIBIB program director in Interventional Ultrasound.
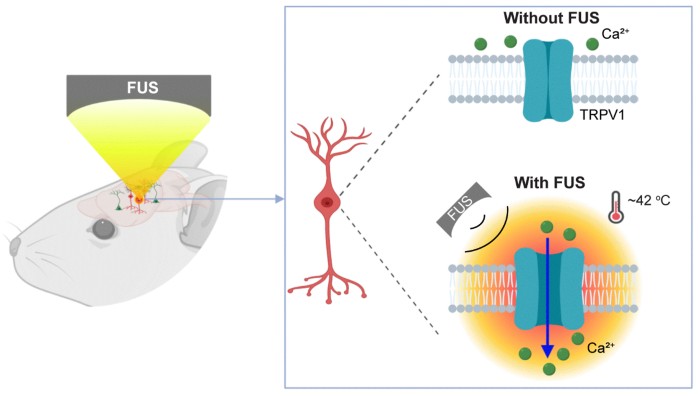
The work was made possible by combining ultrasound and a heat-sensitive cell surface ion channel called TRPV1. Tests on cell cultures confirmed that when TRPV1 is on the surface of cells, ultrasound treatment increases the temperature by only 4 to 5 degrees, causing the TRPV1 ion channel to activate.
The group then tested their system on mice. They used a viral vector carrying the TRPV1 gene to deliver the heat-sensitive ion channel to the striatum of the brains of mice. The striatum was chosen because it is the area of the brain that controls motor movement.
Focused ultrasound was then performed on the striatum. When the focused ultrasound was turned on, the TRPV1 channel was activated and the mice repeatedly spun in circles until the ultrasound was turned off. There were no changes in movement in ultrasound-treated mice that had not been infected with the viral vector and therefore did not have the TRPV1 gene in striatal neurons. The results confirmed that infecting the desired neuronal cell type with the heat-sensitive TRPV1 gene allowed the researchers to influence the activity of the desired brain cells with focused ultrasound.
The research team is now working to expand sonothermogenetics from mice to larger animal models. As a proof of concept for potential use in humans, the team has begun work using the sonothermogenetic system to modulate eye movements in non-human primates.
“It will take us a long way to translate this technique to humans because it involves genetic engineering to modify the neurons in the brain,” explains Hong, about the potential of sonothermogenetic technology. “But we are confident that once we overcome all the obstacles and translate this technique into the clinic, it will play a transformative role in next-generation therapies for the treatment of diseases of the central neural system, such as Parkinson’s, depression and epilepsy.”
The results of the study were published in the journal Brain Stimulation. [1].
The work was supported by the BRAIN Initiative (R01MH116981) and NIBIB (R01EB027223 and R01EB030102) of the National Institutes of Health (NIH), and the Hope Center Viral Vectors Core of the University of Washington School of Medicine.