Biomedical imaging modalities, such as magnetic resonance imaging (MRI), have revolutionized the ability to detect and track the progress of many types of cancer. However, the difficulty in obtaining detailed images of cancer cells buried deep in normal tissues has slowed the usefulness of imaging technology for improved and personalized cancer care.
One approach that biomedical engineers are pursuing to overcome this limitation involves building nanoprobes that are designed to travel throughout the body, accumulate in cancer cells, and send a signal that illuminates the tumor.
Johns Hopkins University researchers supported by NIBIB have developed a smart nanoprobe designed to infiltrate prostate tumors and send a signal using an optical imaging technique known as Raman spectroscopy. The new research, reported in Advanced sciencehas the potential to determine tumor aggressiveness and could also allow sequential monitoring of tumors during therapy to quickly determine whether a treatment strategy is working.
“We are excited about the potential to improve the diagnosis and treatment of many common cancers,” explained project leaders Jeff Bulte, Ph.D., and Ishan Barman, Ph.D. “We are combining self-assembling nanoprobes with Raman spectroscopy to achieve precise imaging with single-cell resolution necessary for eventual practical use in the clinic.”
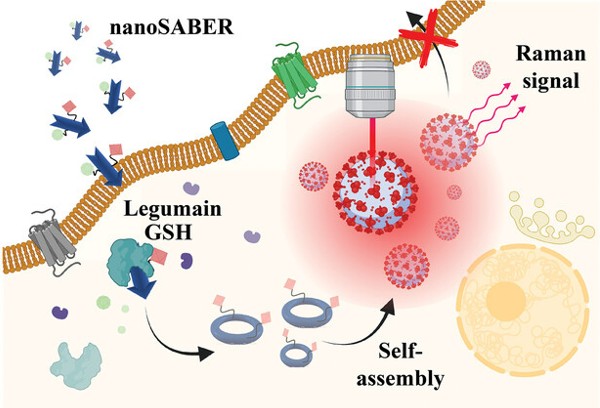
The engineering team built a nanoprobe that is sensitive to its local microenvironment: it activates only after encountering leguminous, a tumor-associated enzyme produced by aggressive prostate cancer cells. Once it finds the nanoprobe, legumain splits it into pieces that can self-assemble to create an optically active nanoparticle. These nanoparticles emit specific wavelengths of light that can be detected with Raman spectroscopy to visualize the tumor.
Postdoctoral fellows Swati Tanwar, Ph.D., and Behnaz Ghaemi, Ph.D., led the design and synthesis of the nanoprobe, called nanoSABRE (for Self-Assembling Bioorthogonal Enzyme Recognition), an apt name that reflects the smart molecule’s surgical precision.
“We have chosen Raman reporters that are specifically active in the ‘cellular silent’ region of the near-infrared spectrum to avoid interference with the normal tissue signal,” Barman explained. “This selective activity is crucial for our imaging technique, as it allows accurate detection using Raman spectroscopy without reacting or being obscured by surrounding biological material.”
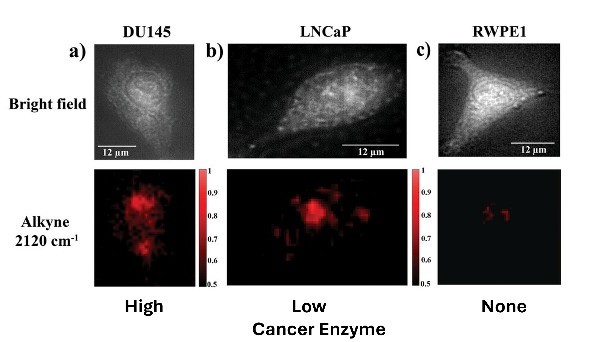
The researchers used two prostate cancer cell lines that exhibited varying levels of leguminous expression (one high, one low) along with a non-cancerous prostate epithelial cell line, which produces a negligible amount of the enzyme.
NanoSABER was tested in laboratory cell cultures and in an experimental mouse model. In both environments, leguminous-expressing prostate cells activated the nanoprobe and emitted a signal with an intensity that corresponded to the amount of leguminous produced by the cancer cells. The non-leguminous cell type did not activate the nanoprobe, demonstrating that the nanoSABER system worked as designed, emitting a signal that correctly indicated the presence and quantity of the cancer-associated enzyme.
“This group’s work is an important step toward better care for men with prostate cancer,” explained Tatjana Atanasijevic, Ph.D., program director of NIBIB’s Division of Applied Science and Technology. “It is an excellent example of the type of innovative technologies that NIBIB supports that have the potential to have a dramatic impact on healthcare.”
The team believes they have designed a molecular system with the potential not only to identify tumors using optical imaging, but also to rapidly assess tumor aggressiveness, potentially without the need for painful biopsies that are the current standard of care. Additionally, as enzyme secretion profiles by different types of cancers are discovered, additional nanoSABER probes may be synthesized that will enable a level of accurate diagnosis of tumor types and characteristics that is not currently possible, including sequential imaging of tumors to determine whether therapies are working in real time.
This work was supported by grants from NIBIB (R01EB030376, P41EB015871, and P41EB024495), the National Cancer Institute (NCI; R01CA238025), and the National Institute of General Medical Sciences (NIGMS; R35GM149272 and DP2GM128198).
This prominent scientist describes a basic research finding. Basic research increases our understanding of human behavior and biology, which is critical to promoting new and better ways to prevent, diagnose, and treat diseases. Science is an unpredictable and incremental process: each research advance builds on past discoveries, often in unexpected ways. Most clinical advances would not be possible without knowledge of fundamental basic research.
Study Reference: A Smart Intracellular Self-Assembling Bioorthogonal Raman Active Nanoprobe for Targeted Tumor Imaging. Tanwar S, Ghaemi B, Raj P, Singh A, Wu L, Yuan Y, Arifin DR, McMahon MT, Bulte JWM, Barman I. Adv Sci (Weinh). December 2023; 10 (34): e2304164. doi: 10.1002/advs.202304164.
About the graphs: Both graphs used in this highlight were adapted from the figures in the publication in Advanced science and are licensed under the Creative Commons Attribution 4.0 International License.