Biological drugs (so called because they are usually isolated from a living source, rather than chemically synthesized) are used to treat a wide variety of conditions, including diabetes, inflammatory diseases, and certain types of cancer. However, due to their complex and easily degradable components, administration of these medications often requires self-injection, which can pose a burden to patients, including the training required for proper self-administration and the potential for needle sticks and pain. Now, NIBIB-funded researchers are developing a robotic pill that, after swallowing, can deliver biological drugs to the stomach, potentially revolutionizing the way certain conditions are treated.
“Due to the inherent drawbacks of injectable medications, many healthcare professionals prescribe less effective oral medications instead, resulting in suboptimal treatment for many patients,” said David Rampulla, Ph.D., division director of Discovery Science & Technology at NIBIB. “An oral pill for biologic drug delivery would not only have a positive impact on those patients already using injectable medications, but could also benefit patients who are currently delaying their use. This preclinical research is an important step toward developing such an approach.”
The study authors built on previous research in which they developed robotic pills that were capable of delivering solid biological drugs to the systemic circulation by injection into the stomach and small intestine. These devices, evaluated in animal models, supported lower drug doses (about half a milligram per capsule), which would require frequent oral doses for some commonly used biologic drugs. They also had limited immediate absorption of the drug, precluding its use for rapid-acting purposes (such as mealtime insulin). “We applied lessons learned from our previous work and developed a new device that can inject a hollow needle into a shallow layer of the stomach lining, allowing for the administration of liquid medications,” explained the study’s senior author, Giovanni Traverso, M.D., Ph.D, assistant professor in the Department of Mechanical Engineering at the Massachusetts Institute of Technology. “Administration of a liquid formulation allows absorption and effects of the drug within five minutes of ingesting the capsule,” said Traverso, who is also an associate physician at Brigham and Women’s Hospital and an assistant professor at Harvard Medical School.
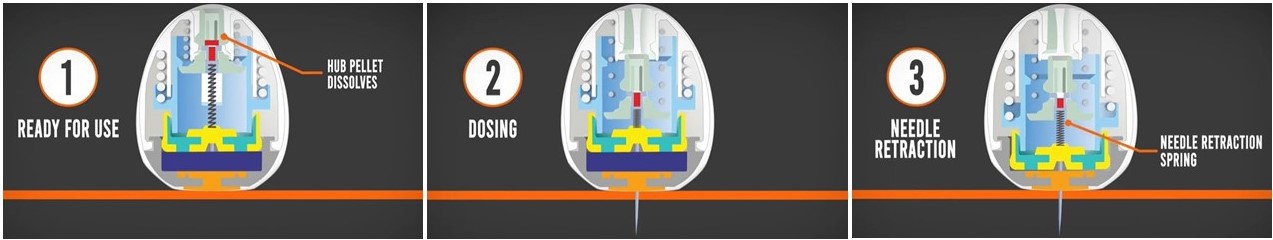
He explained how his improved device works like a domino: Once the pill has been swallowed and reaches the stomach, it uses its weighted base to orient itself properly so that its injection mechanism sits flush with the stomach wall. After a few minutes, a carbohydrate pellet at the top of the pill dissolves, activating a spring and allowing a needle to inject the biologic drug into the stomach tissue. Then, a second, newly exposed pellet dissolves, releasing the spring and retracting the needle back into the pill, allowing safe passage of the device through the gastrointestinal tract.
“For the shape of our robotic pill, we were inspired by wobbling toys and leopard tortoises, which have a wide, heavy bottom that allows them to right themselves,” said study author Ulrik Rahbek, Ph.D., vice president of Partnerships and Portfolio in Research and Early Development at Novo Nordisk, a Danish multinational pharmaceutical company. “This ensures that the biologic drug is successfully delivered into the stomach tissue rather than into the stomach cavity, which is full of enzymes that can easily degrade and inactivate these sensitive drugs.”
In their study, reported in Nature BiotechnologyThe authors loaded their robotic pills with one of four biologic drugs: insulin; a glucagon-like peptide 1 (GLP-1) analog (also used for the treatment of diabetes); adalimumab (brand name Humira®, an immunosuppressive drug used for a variety of conditions, including arthritis and Crohn’s disease); or epinephrine (for emergency treatment of allergic reactions or asthma attacks). Using an endoscope, the researchers placed the robotic pills loaded with the drug into the pigs’ stomachs and allowed them to self-activate. Then, after 15 minutes or two hours (depending on the drug), the pills were removed. The researchers evaluated drug exposure in the animals’ blood along with other relevant metrics (such as the occurrence of hypoglycemia in insulin treatment or increased heart rate in epinephrine treatment).
In the 31 large animals in this preclinical study, 28 demonstrated systemic absorption of the dosed biological drug, resulting in a positive dosing rate greater than 90%.
To evaluate how well the robotic pills worked compared to traditional biologic drug administrations, the researchers gave separate pigs the same four drugs, either via subcutaneous (fatty tissue) or intramuscular injections. They found that delivering treatment using robotic pills resulted in drug exposures similar to delivering treatment using traditional injection methods for all four drugs tested.
“We found that our device produces drug absorption comparable to current injection methods and can facilitate drug exposure within minutes,” Traverso said. “Combined with the fact that our capsule can deliver large doses (up to four milligrams) of complex biologic drugs, our robotic pill could have a transformative impact on patients who rely on injectable medications.”
To evaluate whether the robotic pills could be ingested and pass through the gastrointestinal tract without difficulty, the researchers evaluated similar robotic pills (without any medication) in eight dogs. They found that all animals were able to ingest the capsule with ease, and radiographic monitoring revealed that the devices traveled to the stomach, were activated, and then traveled through the gastrointestinal tract as expected.
The study authors emphasized the preclinical nature of the current study and that testing in humans has not yet been performed.
“Although there is still much work to be done, this capsule has the potential to offer an alternative to injections in a variety of therapies,” concluded Rahbek.
Traverso and his colleagues are also investigating their robotic pills for the delivery of therapeutic nucleic acids, such as mRNA vaccines, in animal models. The first works have just been published in Affair.
The studies referenced in this paper were funded in part by a NIBIB grant (EB-000244).
Study reference: Abramson, A., Frederiksen, M.R., Vegge, A. et al. Oral administration of systemic monoclonal antibodies, peptides and small molecules using gastric autoinjectors. Nat Biotechnol 40, 103-109 (2022). https://doi.org/10.1038/s41587-021-01024-0