NIBIB-funded Ohio State University (OSU) biomedical engineers have demonstrated a new method of delivering an anti-cancer drug in a study that tested the effect in animal models. When formulated inside a membrane sac, called an exosome, and when combined with the B vitamin folate, the anticancer drug can enter the cell without being sealed inside the cell by another sac, called an endosome. Endosome capture has been a formidable challenge to overcome in drug delivery.
The approach, developed with support from the National Institute of Biomedical Imaging and Bioengineering (NIBIB) and the National Cancer Institute (NCI), both at the NIH, works on the principle that folate receptors are expressed abundantly in cancer cells, but to a more moderate degree in healthy cells. Reporting in the August 22, 2019, online edition of the Journal of Controlled Release, the authors suggest that their approach, tested in mice, will renew interest in folate as a broad target for human cancer therapy.
“Targeting folate has been an elusive approach to cancer therapy,” said David Rampulla, Ph.D., director of NIBIB’s Division of Discovery Science and Technology. “This team has demonstrated an efficient drug delivery system and shown how a nanoparticle combined with folate can effectively target cancer cells. It is the type of advance that could represent the basis for much-needed cancer therapies.”
Folate is a B vitamin necessary for cell synthesis and division. Due to the increased expression of folate receptors on cancer cells, folate has been widely proposed as a targeted cancer therapy for 25 years, having been extensively tested in studies with breast, lung, ovarian, colorectal, and head and neck cancers. Typical approaches combine folate with an anticancer drug, such as an RNA interference nanoparticle, which has the potential to alter the genetic machinery within cancer cells.
Ideally, folate is recognized by receptors on the cell membrane, allowing the anti-cancer nanoparticle to access the cell. However, researchers have run into the challenge that treatments administered through the folate receptor pathway become trapped within the endosome sac. Therefore, in the past, the use of folate for the delivery of specific drugs has not been successful.
The OSU team, led by senior author Peixuan Guo, Ph.D., the Sylvan G. Frank Chair in Pharmaceutical Drug Delivery, applied an alternative approach to delivering the interfering RNA into the cell. They placed RNA interference nanoparticles on an exosome with folate on its surface. Upon contact with the cancer cell membrane, the exosome specifically binds and fuses with the cancer cell membrane, releasing its therapeutic content into the aqueous component of the cytoplasm (cytosol).
The research team had previously shown that the approach could be effective in preventing breast, colorectal and prostate cancers in mice. That study was published December 11, 2017 in Nature Nanotechnology.
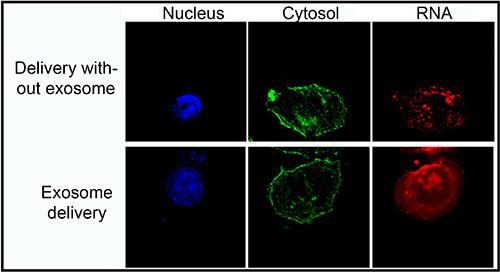
In their current study, the researchers took the next step and conducted tests to better elucidate the method of delivering the treatment. To show that folate receptors enhance the delivery of RNA interference nanoparticles when administered as a component of the exosome, they combined folate with an RNA interference called survivin siRNA, which disrupts a protein in cancer cells that can inhibit cell death. Cancer occurs when cells do not die and proliferate uncontrollably. They used fluorescent optical imaging technology to capture the effect of delivering the treatment through an exosome compared to delivering survivin siRNA without an exosome. The images showed that exosome administration allowed the treatment to distribute throughout the cell.
To determine whether folate receptors on cancer cells can be specifically targeted, the team applied the treatment to cervical cancer in mice. Mice treated with folate and survivin siRNA bound within an exosome reduced tumor growth, confirming the efficacy of the new cancer treatment approach. “The therapeutic effect is surprisingly high,” Guo said. “This finding will be a revolution to renew an outdated concept about the use of folate as a specific agent in cancer therapy.”
The team received support from NIBIB (EB019036) and NCI (CA151648, CA207946, CA186100, CA 168505, CA209045) to conduct this research.
Article: Cytosolic siRNA delivery mediated by folate-displaying exosomes avoiding endosome trapping. Zheng Z, Li Z, Xu C, Guo B, Guo P. Journal of Controlled Release. August 22, 2019.