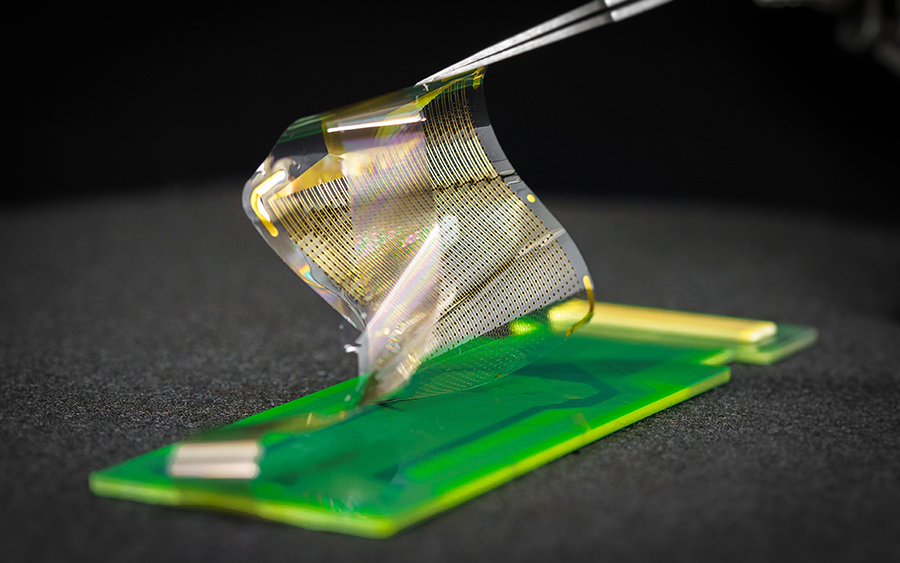
Improvements in brain sensing technologies have allowed doctors to perform increasingly complex surgeries and researchers to map the brain signals that control feelings, movement and thinking. Now, a multidisciplinary research team has designed, manufactured and tested the latest in brain probing technologies that promises to unlock the brain’s functional networks in unprecedented detail.
To obtain functional signal maps, brain probing technologies often consist of electrode grids placed directly over the brain as a small network. This type of grid with sensors to record signals is known as electrocorticography (ECoG). During surgery to remove tumors or epileptic regions, signals emanating from the grid guide the removal of diseased tissue while helping surgeons avoid damage to surrounding healthy tissue. The research uses grids placed in the brains of animals and humans to record and map signals that carry out specific functions such as reaching and grasping.
Despite these notable achievements, unraveling the complexity of the brain has only just begun.
To address the challenge of mapping the brain with increasing precision, a team of engineers, medical researchers and surgeons has harnessed the power of nanotechnology to build networks that can record signals from the human brain with significantly greater precision than current technologies. The project is led by electrical engineering professor Shadi Dayeh of the Jacobs School of Engineering at the University of California, San Diego, and includes team members from UC San Diego, Massachusetts General Hospital, and Oregon Health and Science University.
New nanotechnology-based networks are made up of several thousand sensors, compared to current ECoG networks, which typically have between 16 and 64 sensors.
The technology is called platinum nanorod grating (PtNRGrid). The PtNRGrid creates brain maps that are 100 times more accurate because the tiny nanosensors can be densely packed (placed just a millimeter apart) compared to current grids with sensors spaced at centimeter intervals.
“A central problem in electronics and sensors is that when placed too close to each other, one sensor interferes with the signal of the other,” explained Moira Bittman, Ph.D., director of the Biorobotic Systems program at the National Institute of Biomedical Imaging and Bioengineering (NIBIB), which co-funded the research. “The team has used elegant engineering and manufacturing techniques that allow these powerful sensors to operate precisely while in extremely close contact. It is a great example of the type of innovations we support at NIBIB that are poised to drive significant advances in research and medicine.”
The team tested the new technology on rats and on humans undergoing brain surgery.
A corn kernel-sized PtNRGrid containing 1024 sensors was placed in a small region of a rat’s brain. Small puffs of air were used to stimulate sensory areas of the rat, such as the whiskers and skin. When a puff of air came into contact with a single whisker, the grid picked up the electrical signal in the area of the brain that controls sensations from that exact whisker. The PtNRGrid produced a brain map that identifies the area of the brain connected to each of the 16 individual whiskers separated by just fractions of a millimeter, a level of detail that was not possible with previous technologies.
A postage-stamp-sized PtNRGrid containing 1024 or 2048 nanosensors was also used in human experiments. The patients had given permission to conduct the research studies while undergoing surgery for tumors or to remove regions of the brain that caused epileptic seizures.
The 1024 PtNRGrid sensor identified the brain region that controls sensation in each of the five fingertips in the test subjects, as well as the regions that control the grasping movement. Surprisingly, the grid was able to capture the different brain signals involved in the planning stage of the movement, the actual movement, and after the completion of the movement – signals that occur just a millionth of a second apart.
In tests on a patient with epilepsy using 2048 sensors, the PtNRgrid was able to identify the location of areas of the brain that discharge epileptic waves with millimeter precision, as well as test waves that the researchers induced in different areas of the brain. The millimeter resolution of the areas that identify where the signals originated was higher than the centimeter resolution that can be obtained with the technologies currently used. This level of precision could allow the surgeon to remove diseased tissue just millimeters away from healthy tissue instead of using a margin of centimeters, thus saving more function.
{“preview_thumbnail”:”/sites/default/files/styles/video_embed_wysiwyg_preview/public/video_thumbnails/-7ggs6e2UXI.jpg?itok=BBjQgNIq”,”video_url”:”https://youtu.be/-7ggs6e2UXI“,”settings”:{“responsive”:1,”width”:”854″,”height”:”480″,”autoplay”:0},”settings_summary”:[“Embedded Video (Responsive).”]}
“We are excited and excited about the possibilities this new technology offers,” Dayeh explained. “We estimate that hundreds of thousands of people would benefit from the significantly more precise brain surgeries that will be possible using PtNRGrids. Additionally, our process for manufacturing the grids is similar to that used to manufacture television, phone and computer screens, allowing us to efficiently add functional utility to the grids.”
By “functional utility,” Dayeh explains that the team is working to produce cost-effective grids that will provide even more accurate and useful information to surgeons as they perform these extraordinarily difficult procedures.
The study was published in Science Translational Medicine.1. The work was supported by NIH award no. NIBIB DP2-EB029757 to SAD, the BRAIN® Initiative grants R01NS123655-01 to SAD and UG3NS123723-01 to SAD, and NIDA R01-DA050159 to SAD; NSF award no. 1728497 to SAD and CARRERA no. 1351980 to SAD; and an NSF Graduate Research Fellowship Program no. DGE-1650112 to AMB