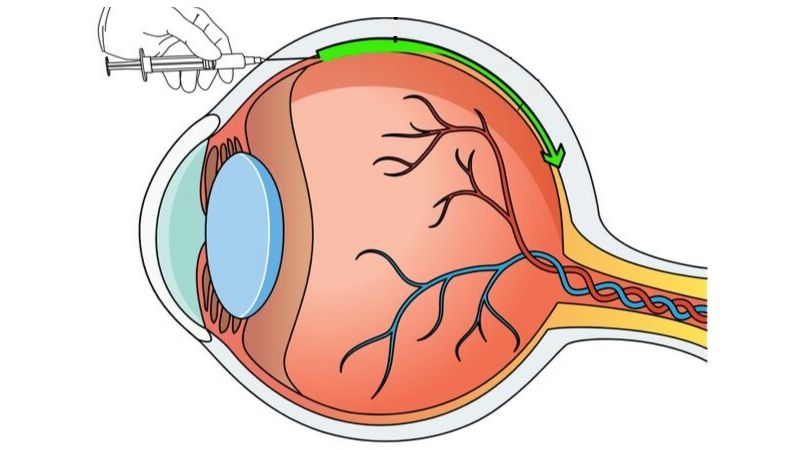
NIBIB-funded researchers have created nanoparticles for successful gene therapy of a mouse model of macular degeneration. Nanoparticle carriers have the potential to significantly expand the growing repertoire and efficacy of gene therapies for human eye diseases, including blindness.
Several eye diseases have benefited from gene therapies in which DNA encoding therapeutic proteins is delivered to cells of the eye. Current therapies deliver DNA using viral vectors, but this method has limitations, including the development of an immune response against the viral carrier and limits to the size of the gene that can fit into the virus.
Engineers and ophthalmologists have come together to address the limitations of viral vectors in the treatment of eye diseases that cause blindness, such as wet age-related macular degeneration (AMD). Wet AMD is a common disease characterized by abnormal blood vessel growth that damages light-sensitive tissue at the back of the eye.
The Johns Hopkins University School of Medicine research team was led by Jordan Green, Ph.D., Professor of Biomedical Engineering, Ophthalmology, Oncology, Neurosurgery, Materials Science and Engineering, and Chemical and Biomolecular Engineering, and Peter A. Campochiaro, MD, Director of the Retinal Cell and Molecular Laboratory and George S. and Dolores Dor Eccles Professor of Ophthalmology and Neuroscience.
“This is an exceptional collaboration that combines Dr. Green’s work in creating nanoparticles for gene delivery and Dr. Campochiaro’s expertise in retinal diseases,” said David Rampulla, Ph.D., NIBIB Program Director in Biomimetic and Synthetic Biological Systems. “The system they have created promises to reduce the debilitating effects of eye diseases and preserve sight by inhibiting disease progression.”
“We have been able to use gene therapy to treat eye diseases because the eye is very accessible and quite easily picks up the viruses that carry the therapeutic gene,” explains Campochiaro. “However, expression of the therapeutic protein eventually fades, requiring repeated injections of the virus carrying the gene to maintain adequate levels of the therapeutic protein.”
Repeated treatments are problematic because they involve frequent eye injections for patients. And subsequent rounds of treatment are often unsuccessful because the patient’s immune system has developed antibodies that attack and neutralize the virus carrying the gene.
That’s where Green’s nanoparticle technology comes into play. The nanoparticles are virtually undetected by the immune system and there is no apparent development of antibodies that would attack the particle in subsequent treatment. Another desirable feature is that nanoparticles can carry therapeutic genes that are too large to be carried by viral vectors.
The team performed a series of experiments in rats and mice that showed that the nanoparticles very efficiently transported genes to the cells of the eye and produced large amounts of proteins. The proteins continued to be produced for at least eight months, a significant improvement over current treatments in which therapeutic proteins must be injected directly every 1 to 2 months.
One test was performed using a gene that produces vascular endothelial growth factor (VEGF). VEGF induces the growth of new blood vessels in the eye. If there is too much VEGF there is an overproduction of blood vessels that extend into the retina and block vision, which is what happens in people with wet AMD.
The researchers injected nanoparticles carrying the VEGF gene into the eyes of 30 rats. The rats developed abnormal blood vessels a month after the injection. The abnormal blood vessels were more extensive at two and five months after the injection, and there were scars under the retina similar to those seen in patients with untreated wet AMD. This validated the efficacy and durability of the gene therapy system for the creation of animal models.
The therapeutic potential of the delivery system was tested by administering a nanoparticle carrying a gene that produces a protein that inhibits VEGF.
The inhibitory nanoparticle was injected into a mouse model of wet AMD, which exhibits the overproduction of abnormal blood vessels seen in the human disease. Three weeks after injecting nanoparticles containing the VEGF inhibitor protein gene, the mice had a 60% reduction in abnormal blood vessels compared to control mice.
“These results are extremely promising,” Green said. “We have shown that nanoparticles have the potential to transmit genes that produce active therapeutic proteins for many months.” The result is a considerable improvement over the current therapy of directly injecting the therapeutic protein, which requires repeated injections approximately every month. “The lack of an apparent immune response to the nanoparticles is also a significant improvement over gene therapy with viral vectors.”
The team is now working on using the nanoparticle system to treat patients with reduced or no vision due to genetic defects in which blindness occurs due to a defective inherited gene. They hope that the robust gene expression seen with their nanoparticles will allow them to introduce functional versions of genes that inherited mutations have disabled and restore vision.
The work appears in the July issue of the journal Science Advances.1.
The research was supported by grant EB022148 from the National Institute of Biomedical Imaging and Bioengineering, grants EY031097, EY026148, EY028996, and EY01765 from the National Eye Institute; the Dr. H. James and Carole Free Catalyst Award and an unrestricted grant from Research to Prevent Blindness; the Louis B. Thalheimer Translational Fund; a grant from the Barth Foundation; a scholarship for JK from Samsung; and unrestricted grants from Conrad and Lois Aschenbach, Per Bang-Jensen, Andrew and Yvette Marriott, and Jean Lake.