Typical diagnosis of upper urinary tract cancers requires removal of suspicious tissue with forceps, a technically challenging procedure that only samples one region of the organ. NIBIB-funded researchers are developing microforceps that could be deployed throughout the upper urinary tract, which could capture small pieces of tissue in hundreds of different areas and potentially facilitate early disease detection. Their preclinical research was recently published in the journal Advanced healthcare materials.
“Many cancer diagnoses result from accidental discovery through imaging or when a patient has pain or bleeding; in other words, when the tumor is large enough to see or when it begins to affect the function of surrounding tissue,” explained the study’s senior author, David Gracias, Ph.D., a professor at Johns Hopkins University. “Our goal is to create a new diagnostic screening paradigm that can find cancer cells before a visible tumor develops, which could allow for earlier detection of cancer and boost treatment.”
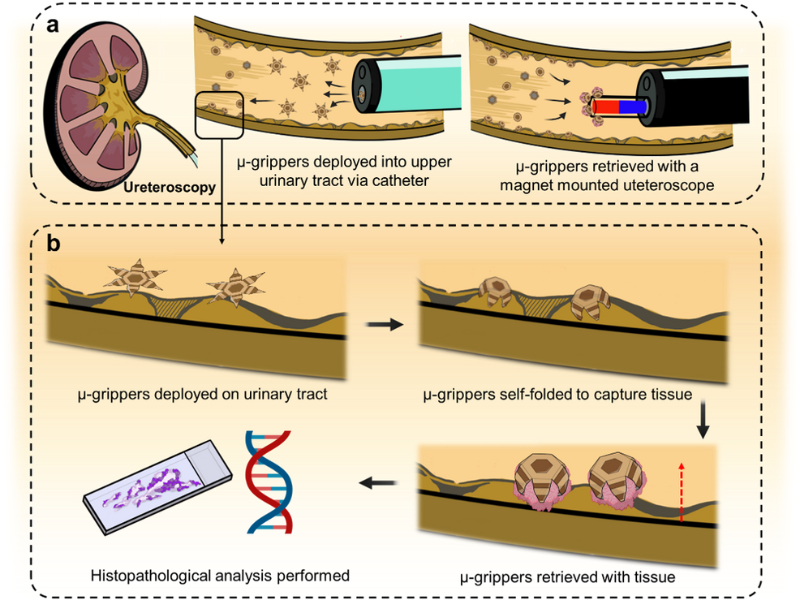
Although Thank you and colleagues have previously developed microforceps for the gastrointestinal tract, in this study they focused on the upper urinary tract, since its location and complex anatomy make obtaining tissue samples somewhat difficult. The ureters, which are the tubes that carry urine from the kidney to the bladder, are less than five millimeters in diameter but can be up to 30 centimeters long. This limits both the size of tools that can be used and the quality and quantity of tissue samples that can be extracted.
Taking these factors into account, the researchers designed their microtweezers to be extremely small: less than 750 microns, or smaller than the tip of a sharp pencil. The microforceps are star-shaped and composed of six arms with multiple hinges, allowing them to fold into a claw shape. After tissue is collected, a magnet can be used to retrieve the microforceps, which can be collectively analyzed to see if any of the samples they brought back are cancerous.
Microtweezers are like a loaded spring, Gracias explained. They can be made by the thousands in sheets with a coating of food-grade wax that keeps them flat. “After exposure to body temperature, the wax softens, releasing the energy of spring,” Gracias said. “This allows the microforceps to bend with enough force to pierce and grasp the surrounding tissue.”
Typical upper urinary tract biopsies use a specialized ureteral catheter, which slides forceps through the urinary tract to collect tissue. To understand whether microforceps could be compatible with this technique, Gracias and his colleagues loaded approximately 200 microforceps into a vial and used compressed air to propel them through three different commercial catheters. They found that all catheter types had similar passage rates, with approximately 80% of microclamps passing successfully.
Gracias and his team then evaluated the microforceps in a ex-live pig ureter. They deployed the microforceps into the removed organ and then incubated the ureter at body temperature for about ten minutes. They then used a magnet to remove the microforceps from the ureter and found that most of the microforceps had grabbed and retrieved small clumps of tissue.
Analysis of the excised tissue revealed that the microforceps were able to retrieve multiple layers of urothelial cells, indicating that this technique can acquire diagnostic quality samples. While each microforceps contains a very small amount of tissue, if hundreds are deployed into the upper urinary tract, the total amount of tissue collected could be similar to a traditional biopsy procedure, Gracias explained. However, the tissue would be collected from many different regions of the organ rather than one localized area.
This factor could make microforceps an effective detection tool. Gracias compared his technique to a city survey: If all the information comes from the same neighborhood, it is not representative of the city’s population as a whole. “We plan to use this sampling method to determine the overall health of an entire organ,” Gracias said. “Doctors could someday use microforceps to detect high-risk patients, potentially finding diseases before symptoms develop.”
While the team is currently focused on the urinary and upper gastrointestinal tracts, the microforceps could be implemented in other organs. “Their small size and clever mechanism of action distinguish these microrobots from other biopsy tools in the field,” said Jessica Falcone, Ph.D., program director of NIBIB’s Science and Technology Discovery Division. “Future work in live animals will help determine whether this technology could one day be translated into a detection tool for humans.”
This study was funded in part by NIBIB grant R01EB017742.
This prominent scientist describes a basic research finding. Basic research increases our understanding of human behavior and biology, which is critical to promoting new and better ways to prevent, diagnose, and treat diseases. Science is an unpredictable and incremental process: each research advance builds on past discoveries, often in unexpected ways. Most clinical advances would not be possible without knowledge of fundamental basic research.
Study reference: W. Liu et al. Untethered microforceps for upper urinary tract biopsy. Adv. Health matter. 2024, 2401407. https://doi.org/10.1002/adhm.202401407