1. Uses an ultrasound device found in most healthcare settings and an inexpensive laser light source.
2. The absence of ionizing radiation allows repeat imaging to monitor the tumor’s response to treatment.
3. Nanoparticle contrast agent can be designed to target different types of tumors.
Photoacoustic (PA) imaging is a non-ionizing imaging platform that combines light and ultrasound to safely image structures and molecules in the body. Researchers have now designed a nanoparticle-based PA contrast agent that targets and significantly improves photoacoustic imaging of ovarian tumors in a mouse model. Completely constructed from FDA-approved components used in other medical procedures, the technology was specifically designed to accelerate its use in humans to detect ovarian and other cancers.
AP imaging uses light and sound instead of radioactivity to image organs and tissues at the molecular level. The system sends pulses of near-infrared (NIR) light through the skin and into tissues. The light heats and expands molecules in the target tissue causing vibrations that bounce back and can be detected using ultrasound to generate an image.
High-quality PA images can be obtained using contrast agents that target specific molecules, such as receptors commonly overexpressed by cancer cells. Contrast agents are highly light-sensitive molecules that are systemically infused into a patient and bind to the surface of the tumor. When exposed to pulsed light, the contrast agent creates tiny vibrations that can be detected using ultrasound to provide molecular images suitable for clinical applications such as tumor detection.
To date, the only clinically approved imaging system that is also capable of detecting molecular changes in tissues and organs is positron emission tomography (PET), which uses ionizing radiation and complex, expensive machinery, limiting its use for widespread and repeated imaging of cancer. Ionizing radiation can cause tissue damage, especially if a patient is exposed repeatedly.
Because it does not use ionizing radiation and the equipment required is much less expensive than PET imaging devices, PA imaging is poised to fill an “imaging gap.” That gap exists in clinics and healthcare centers that don’t have expensive PET imagers, but often already have the ultrasound machines needed to obtain AP images. These clinics, with the addition of an affordable laser that generates the necessary light pulses, would be prepared to offer point-of-care cancer imaging to patients at local sites. In particular, PA imaging is ideal for patients undergoing cancer treatment, as the non-ionizing technique can be performed repeatedly to monitor a tumor’s response to therapy.
Unfortunately, this promising imaging system has been slow to move from the laboratory to the clinic. To address this problem, researchers at The University of Texas MD Anderson Cancer Center are working to develop a molecular contrast agent for PA imaging that is ready for the clinic and has the potential to detect breast, ovarian, prostate, and other types of tumors.
“This research was driven by two important goals,” explained Richard Bouchard, MD Anderson’s Department of Imaging Physics and co-senior author of the study. “First, design and synthesize a nanoparticle contrast agent with all the features necessary to generate a high-contrast image of cellular receptors overexpressed by cancer cells at clinically relevant depths, which would be centimeters beneath the skin. Second, construct that nanoparticle entirely from components that are already approved by the FDA to avoid regulatory hurdles that have significantly hindered the introduction of this technology into the clinic.”
Necessary features of the nanoparticle envisioned by Bouchard and his colleagues included the ability to reach and bind to molecules of interest, such as specific proteins found in certain tumors. The particle also had to optically absorb enough so that the PA signal was strong enough to bounce through the body and skin and be clearly detected by an ultrasound imaging detector. Optical absorbance had to occur when a narrow wavelength of near-infrared light was used to avoid nonspecific stimulation of surrounding tissues that create noisy and blurry images. Finally, the individual parts of the nanoparticle had to be pre-approved by the FDA for human use.
“This is a comprehensive effort that brings together expertise in chemical synthesis, nanotechnology, photonics, and ultrasound technologies,” said Tatjana Atanasijevic, Ph.D., who directs the Molecular Probes and Imaging Agents program at the National Institute of Biomedical Imaging and Bioengineering (NIBIB), which co-funded the project along with several additional institutes at the National Institutes of Health. “The work goes a long way toward bringing this valuable technology closer to use in the clinic.”
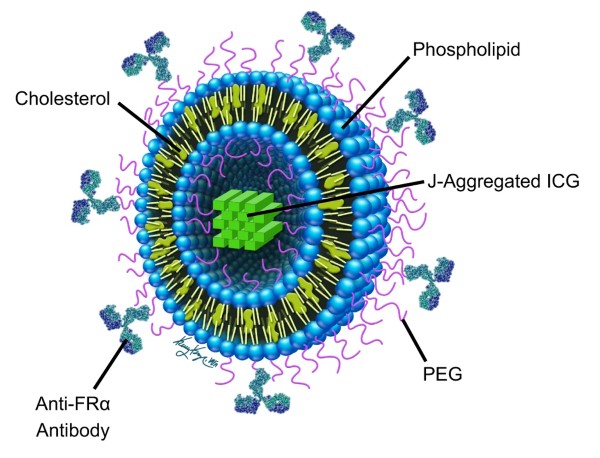
A dye called indocyanine green (ICG), which is already used in humans for imaging, was selected as the contrast agent housed in the center of the particle. However, ICG creates a relatively weak signal, so the team synthesized an aggregate of multiple ICG molecules. Such aggregation results in greater generation of PA signals for imaging deeper tissues.
Furthermore, the ICG aggregate generates a strong BP signal at a narrow NIR wavelength of 890 nanometers, which avoids the problem of confounding BP signals generated by surrounding tissues, especially hemoglobin in the blood. Because blood vessels permeate all organs and are the conduit for systemic delivery of such nanoparticles, it was critical that their agent could be reliably differentiated from hemoglobin for high-contrast imaging.
To test the nanoparticle, the team used a mouse model of ovarian cancer. Getting the systemically introduced nanoparticle to lodge and bind to ovarian tumors was achieved by adding an antibody to the surface of the particle. The antibody specifically binds to a molecule overexpressed on the surface of ovarian tumors called folate receptor.
Finally, all the pieces were packaged into small nanospheres constructed from cholesterol and other lipids already found in the body and routinely used for drug delivery. The complete “stealth” molecule was called PAtrace.
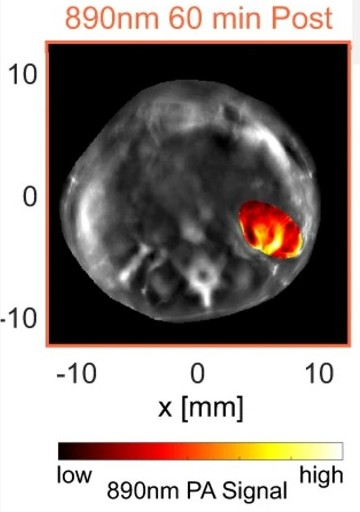
PAtrace was tested in a mouse model of ovarian cancer, which contains human ovarian cancer cells that have been seeded into the mouse ovaries. The nanoparticle was infused into the mouse’s circulation, where it flowed into the ovaries and was small enough to escape the vasculature and bind to nearby ovarian cancer cells. PA imaging was performed and PAtrace generated a clear image of the BB-sized tumors in the mouse ovaries. As designed, the hemoglobin in the surrounding blood vessels did not “light up,” allowing a clear image of the tumors.
“Our PAtrace tests revealed that the components we chose performed extremely well in terms of the various parameters needed to make this type of imaging a viable option,” explained Konstantin Sokolov, MD Anderson’s Department of Imaging Physics and co-senior author of the study. “We are now testing more synthesized PAtrace with new target molecules on the surface of the nanoparticle that will harbor and bind to tumors in various organs such as the breast. The ability to ‘swap’ target molecules on the surface adds versatility to the system as we work toward its eventual use in humans.”
This work was supported by Grant R01EB028762 from the National Institute of Biomedical Imaging and Bioengineering, the National Cancer Institute, the Office of the Director of the National Institutes of Health, the American Cancer Society, the Frank McGraw Memorial Chair in Cancer Research, the Sao Paulo Research Foundation, and the Texas Cancer Prevention and Research Institute. The manuscript appears in the September issue of Nature Communications1.
—Thomas Johnson, Ph.D., special to NIBIB