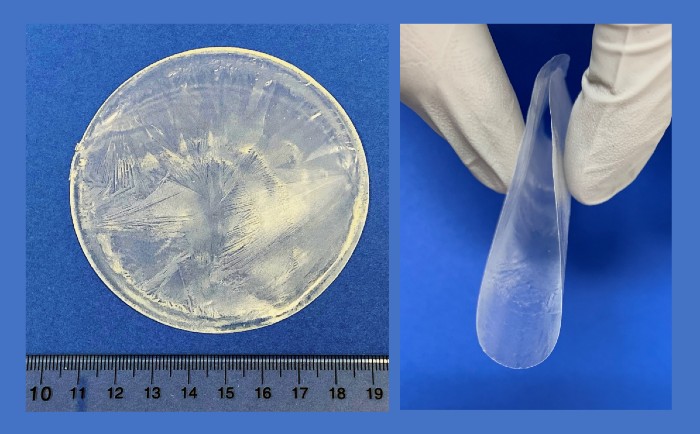
Bioengineers have developed biocompatible generators that create electrical pulses when compressed by body movements. The generators are composed of self-assembling “piezoelectric wafers” that can be manufactured quickly and inexpensively to enable widespread use of muscle-driven electromechanical therapies.
Piezoelectric materials such as ceramics and crystals have the special property of creating an electrical charge in response to mechanical stress. They are used in numerous devices, including ultrasound transducers, vibration sensors, and mobile phones. In medicine, electrostimulation using piezoelectric devices has been shown to be beneficial in accelerating the healing of wounds and bone fractures, maintaining muscle tone in stroke victims, and reducing chronic pain. However, lack of biocompatibility, leading to rigidity and toxicity, has stalled progress in this field.
Now, bioengineers at the University of Wisconsin’s Department of Materials Science and Engineering have developed implantable piezoelectric therapeutic devices. The flexible, wafer-thin devices take advantage of the fact that non-rigid, non-toxic biological materials, such as silk, collagen, and amino acids, also have piezoelectric properties.
The team, led by Xudong Wang, PhD., professor of Materials Science and Engineering, created a method for self-assembly of small patch-like constructs that use the amino acid lysine as a piezoelectric generator. The self-assembly process incorporates a biocompatible polymer shell that surrounds the lysine as the polymer/lysine solution evaporates. The chemical interaction between the inner lysine layer and the polymer coating is critical for orienting the lysine into the crystal structure necessary for it to produce electrical current when flexed.
“This work is an outstanding example of using the chemical properties of materials to create a self-assembling product,” explains David Rampulla, director of the Science and Technology Discovery Division at the National Institute of Biomedical Imaging and Bioengineering, which supports the research. “The process used is fast and inexpensive, making the production of such wafers feasible for therapeutic applications. The fact that the wafers are biodegradable opens up the possibility of creating electrotherapies that could be used to accelerate the healing of an injured bone or muscle, for example, and then degrade and disappear from the body.”
The team performed a series of tests on the properties of the piezoelectric wafers. Wafers were placed on the rats’ legs and chest. Leg and chest movements compressed the piezoelectric wafers enough to create an electrical output. Blood tests performed after the transplanted wafer dissolved in the rats showed normal levels of blood cells and other metabolites, indicating that there were no harmful effects from the dissolved implant.
Wang emphasizes the simplicity of the team’s elegant work that can turn muscle movements into potentially revolutionary therapeutic approaches. “We believe the technology opens up a wide range of possibilities, including real-time sensing, accelerated healing of wounds and other types of injuries, and electrical stimulation to treat pain and other neurological disorders. Importantly, our rapid self-assembly technology dramatically reduces the cost of such devices, which has the potential to greatly expand the use of this very promising form of medical intervention.”
The results were reported in the journal Science. [1].
This work is supported by the National Institute of Biomedical Imaging and Bioengineering under award numbers R01EB021336 and R21EB027857. The small animal imaging facility is supported by the National Cancer Institute.