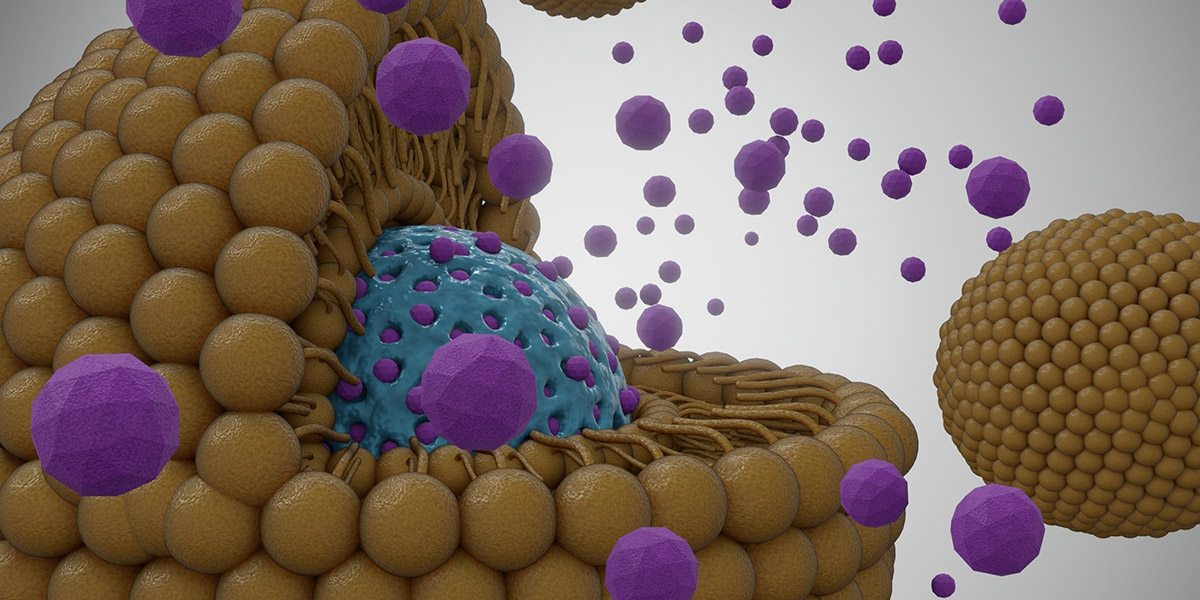
While ultrasound has been widely used for medical imaging, it also has a variety of therapeutic applications. The technology could potentially facilitate the release of drugs in precise locations for disorders and conditions that require drug treatment.
Ideally, a drug would be released only in specific regions of interest and at a high concentration, to maximize benefits and minimize side effects. However, selectively releasing a drug to specific sites in the body, including the brain, has been a challenge. Researchers have addressed the problem by designing ultrasound-sensitive nanoparticles that release a drug at the target site when activated by focused ultrasound.
in a proof of concept studyResearchers at the University of Utah tested whether this method could deliver a drug to a specific area of the brain of non-human primates. The results, published in the Controlled Release Magazine, demonstrated that ultrasound-sensitive nanoparticles delivered a substantial dose of the anesthetic propofol into specific deep regions of the brain. The treatment was found to be safe and effective, and the result was reversible.
“The main benefit of using ultrasound-sensitive nanoparticles is that they encapsulate the drug so that it has minimal interaction with the body, except when released by focused ultrasound,” said Jan Kubanek, Ph.D., assistant professor of biomedical engineering at the University of Utah and corresponding author of the study.
“This could allow us to treat underregulated or malfunctioning brain circuits without exposing the entire brain and body to drugs,” he added.
Designing nanocarriers to deliver a drug payload
The researchers designed new nanoparticles with three layers: an inner core formed by a contrast agent that responds to ultrasound activation, a second layer that encapsulates the drug, and an outer layer..
The design is based on previous research in rodents which demonstrated that nanoparticles can contain contrast agents that change from liquid to gas when interacting with high-frequency ultrasound waves. This approach facilitates a controlled release of the drug at a precise location.
However, a limitation of previous research was that the nanoparticles were unstable when they entered the bloodstream, raising safety concerns.
Kubanek’s team increased the stability of the nanoparticles by choosing a different contrast agent and adding an outer layer to the design. They also encapsulated the drug to prevent it from interacting with surrounding tissues and organs until it was activated by ultrasound and released into the targeted brain region.
Evaluation in large animal models.
The researchers loaded the nanocarriers with a low dose of propofol (an anesthetic that suppresses neural circuits) to evaluate the safety and effectiveness of their combined method. They chose propofol because the drug is used clinically, causes well-defined neuronal inhibition, and its effects on brain circuits occur quickly. This allowed the researchers to test whether the nanocarriers released the drug as intended.
They used an established visual choice experiment to determine whether releasing propofol into specific visual regions of the brain would affect the monkeys’ behavior. Briefly, animals are shown two targets: a flash of light to the left and to the right. They indicate which target appeared first by making an eye movement in that direction.
The researchers focused on the right and left lateral geniculate nuclei, or LGN, which are small structures in the brain that are important for vision. Because each NGL receives input from the opposite side, the researchers expected that inhibiting the NGL on one side would affect vision on the other side. For example, administering propofol directly into the right LGN would impair the animal’s visual perception on the left side, and the animal would be predisposed to choose targets on the right side.
After propofol-loaded nanoparticles were delivered into the bloodstream via injection, the researchers delivered 1-minute ultrasound pulses to the right or left LGN and observed how the animals reacted to the stimuli. As predicted, animals chose the right target when propofol was released into the right LGN. The opposite also occurred, with animals choosing the left target when propofol was released into the left LGN.
The researchers concluded that selective release of propofol modulated the subjects’ visual choice behavior, which was specific to propofol and the target side of the brain compared to ultrasound alone.
The researchers also found that a low dose of propofol achieved targeted brain delivery when activated by ultrasound and minimized its interaction with tissues and organs. This could indicate that lower doses of drugs could achieve the desired effect.
Additionally, the study found that the average time the nanoparticles circulated in the blood was approximately 30 minutes, providing a practical time window for human applications.
“This study is important because it demonstrated a safe and effective approach to release drugs on demand in awake and behaving primates, unlike previous studies that used rodents, thus providing a critical step toward future clinical translation,” said Guoying Liu, Ph.D., director of NIBIB’s Division of Applied Science and Technology.
Two limitations of the study were reported: the publication was not validated using an imaging modality and the reported behavioral effects could be subject to behavioral adaptation and potentially other cognitive influences. However, these limitations were mitigated by contrasting drug release at the two brain sites and by contrasting propofol-filled nanoparticles with saline and empty nanoparticles.
Looking to the future
The researchers aim to eventually apply their formulation to a variety of drugs that cause side effects, including chemotherapy.
“One of the main advantages of this approach is that the nanoparticles are designed to carry any drug and release it upon activation by ultrasound, so this system could be used to treat cancer, pain or addiction,” Kubanek said.
In addition to conducting additional testing in non-human primates, the research team is also testing the potential of this targeted delivery approach to deliver chemotherapy in a mouse model of glioblastoma, the most common, aggressive and deadly brain cancer.
This study was funded in part by NIBIB grant R21EB033638. It was also supported by grants from the National Institute of Neurological Disorders and Stroke (NINDS; R00NS100986), the National Institute of Mental Health (NIMH; F32MH123019), and the NIH Office of the Director (OD; S10OD026788).
This prominent scientist describes a basic research finding. Basic research increases our understanding of human behavior and biology, which is critical to promoting new and better ways to prevent, diagnose, and treat diseases. Science is an unpredictable and incremental process: each research advance builds on past discoveries, often in unexpected ways. Most clinical advances would not be possible without knowledge of fundamental basic research.
Study reference: M Wilson et al. Remotely controlled drug release in deep brain regions of human and non-human primates. Controlled Release Magazine, 2024. https://doi.org/10.1016/j.jconrel.2024.04.013