Skeletal muscle (the type of muscle that attaches to bones and allows movement) is the most abundant tissue in the human body. This tissue has the ability to regenerate after minor injuries, a process facilitated by resident muscle stem cells that help build new muscle fibers. However, acute muscle loss (which can occur in serious accidents), progressive muscle loss, or genetic diseases, such as muscular dystrophy, can compromise this tissue repair process. In these cases, patients may experience mobility problems and have a reduced quality of life, underscoring the need for novel methods to help regenerate skeletal muscle.
Because muscle stem cells can repair damaged tissue, research into stem cell-based therapies for muscle regeneration is an active area of research. While this approach is promising, sources of muscle stem cells are extremely limited and growing these cells in the laboratory is currently an expensive and time-consuming process.
Recently, NIBIB-funded researchers at the University of California, Los Angeles (UCLA) identified a drug cocktail that can effectively activate and expand a population of muscle stem cells, which reside in muscle or are isolated from other tissues, such as skin. The researchers found that muscle stem cells were able to heal damaged tissue in three different mouse models: adult mice, aged mice (which have a reduced healing capacity), and a mouse model of muscular dystrophy (which shows severe muscle atrophy).
“A major obstacle in the field of muscle regeneration is the lack of an effective method to generate and proliferate muscle stem cells,” said senior author Song Li, Ph.D., professor in the departments of bioengineering and medicine at UCLA. “Using our drug cocktail, we can efficiently expand this population of cells, harnessing its potential as a therapeutic agent for muscle repair.”
The drug cocktail includes forskolin, a chemical found in plants that increases the production of cyclic AMP, an important signaling molecule in cells. Also included in the cocktail is a small molecule called RepSox, which mediates stem cell differentiation. While both compounds have been used in stem cell research, their combined effect on muscle stem cell proliferation and differentiation was a novel finding in this study, Li said.
Using skin cells isolated from mice, which contain several types of cells, the researchers used their cocktail to selectively expand a population of chemically induced muscle stem cells. These muscle stem cells were then implanted into the muscles of previously injured mice. Four weeks after implantation, researchers assessed how well the injured muscles had healed. In all their models, the implanted stem cells facilitated muscle repair and tissue regeneration.
Li and his colleagues then took their research a step further. Instead of growing muscle stem cells in a dish, they wanted to see if they could activate and expand the mice’s resident muscle stem cell population by administering the cocktail directly into the muscles. The researchers loaded their drug cocktail into nanoparticles, allowing controlled release of the cocktail over a two-week period. These drug-loaded nanoparticles were then injected into the muscles of previously injured adult or aged animals. Compared to mice injected with empty nanoparticles, mice injected with drug-loaded nanoparticles had markedly improved muscle regeneration and function.
The researchers’ approach of proliferating stem cells that already exist in muscles has several advantages. Instead of growing stem cells in a dish and then implanting them into a patient, Li and his colleagues envision a treatment available for muscle repair by directly injecting their drug cocktail into damaged tissue. However, this approach relies on the presence of resident muscle stem cells and could not be used for extensive muscle loss, Li noted.
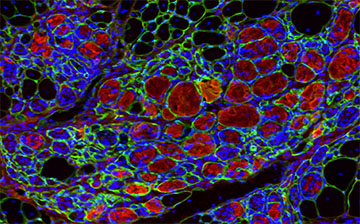
“This work represents a promising step forward in the field of tissue regeneration and could lead to a more efficient method for repairing damaged muscle,” said David Rampulla, Ph.D., director of the division of Discovery Science & Technology at NIBIB. “For patients with muscle injuries or chronic muscle atrophy, this approach could dramatically increase their quality of life,” he said.
Li noted that his cocktail needs further optimization before it can be used with human cells, and that more preclinical studies in larger animal models are needed before this approach can be translated into human clinical therapies.
This study was reported in Nature Biomedical Engineering.
This work was supported by grant EB012240 from the National Institute of Biomedical Imaging and Bioengineering; a grant from the National Heart, Lung, and Blood Institute; a grant from the National Institute of Dental and Craniofacial Research; and by grants from the UCLA Broad Stem Cell Research Center.
This Science Highlight describes a basic research finding. Basic research increases our understanding of human behavior and biology, which is critical to promoting new and better ways to prevent, diagnose, and treat diseases. Science is an unpredictable and incremental process: each research advance builds on past discoveries, often in unexpected ways. Most clinical advances would not be possible without knowledge of fundamental basic research.
Study reference: Fang, J., Sia, J., Soto, J. et al. Skeletal muscle regeneration through chemical induction and expansion of myogenic stem cells in situ or in vitro. Nat Biomed Eng 5864–879 (2021). https://doi.org/10.1038/s41551-021-00696-y