Osteoarthritis, a painful condition that results from the deterioration of the cartilage in our joints, affects millions of people around the world. This persistent problem has been difficult to address, as joint repair techniques, such as cartilage grafts, have major limitations and current tissue regeneration treatments have limited use in human patients. To combat this problem, NIBIB-funded researchers are developing an implantable biodegradable film that helps regenerate native cartilage at the site of damage. Their study, carried out in rabbits, could be an initial and important step in establishing a new treatment for this common disease.
“While most tissue engineering methods rely on growing artificial tissues in the laboratory, this regenerative medicine technique recruits the body’s own cells to facilitate the healing of damaged cartilage,” said David Rampulla, Ph.D., director of the division of Discovery Science & Technology at NIBIB. “This preclinical study, although early, describes a promising approach to regenerating damaged cartilage, which has the potential to benefit those suffering from osteoarthritis and other forms of cartilage damage.”
Traditional surgical treatments for cartilage defects may involve a cartilage graft to replace damaged tissue. These cartilage grafts are taken from another part of the body (from a non-weight-bearing joint, for example) or from a donor. But both options can have potential complications: Grafts taken from another joint can cause additional damage and scarring, while grafts taken from a donor are limited and may be rejected by the body. Other treatments being investigated include growing cartilage on a plate of tissue, but so far this process is time-consuming and has a variety of limitations.
“Rather than treating osteoarthritis by using a cartilage graft at the site of injury, we wanted to develop a therapy that stimulates the regeneration of damaged native cartilage,” explained Thanh Nguyen, Ph.D., associate professor at the University of Connecticut. And it happens that cartilage can regenerate when exposed to a small electrical charge.
But instead of attaching wires or a battery to a damaged joint, Nguyen and his colleagues are using a different approach: Inside the damaged cartilage, they are implanting biodegradable films with piezoelectric properties that generate an electrical charge when compressed. That way, when the joint bears weight from movement or exercise, the film produces an electrical charge that recruits and stimulates cells to regenerate cartilage in the damaged area. The results of this approach were recently published in Scientific translational medicine.
What is piezoelectricity? Piezoelectricity is the ability of a specific material to generate an electrical charge when subjected to mechanical forces, such as pressure. Examples of objects that use piezoelectric components include gas lighters, musical greeting cards, and ultrasound transducers.
In their study, the researchers first evaluated how well their piezoelectric films could improve the chondrogenesis, or cartilage formation, of stem cells grown in the lab. The cells grew on the surface of the piezoelectric film, which was inside a chamber that could be subjected to controllable pressure. The researchers programmed the camera to apply a specific amount of pressure for 20 minutes per day for 14 days. They found that these conditions resulted in increased production of components that make up cartilage, such as collagen and glycosaminoglycans (a type of polysaccharide).
Nguyen explained how the electrical charge stimulated the growth of new cartilage: “We found that cells grown on the piezoelectric scaffolds under pressure had enhanced secretion of an important growth factor involved in wound healing, known as TGF-beta,” he said. What’s more, they found that the charged surface of the piezoelectric film attracted significantly more fibronectin, a protein involved in tissue healing, compared to a non-piezoelectric film. “We believe that the electrical charge generated by our films can recruit proteins important for tissue regeneration and stimulate nearby cells to secrete factors involved in wound healing,” Nguyen said.
The researchers next evaluated their piezoelectric films in rabbits with damaged knee joints, which is one way to model osteoarthritis. The animals were divided into several categories: rabbits with implanted piezoelectric films, rabbits with implanted films that were not piezoelectric, and rabbits without implanted films. Within each category, animals were further classified into groups that participated in an exercise regimen (20 minutes of jumping on a treadmill per day) or did not participate in such exercise, after a month of recovery after surgery.
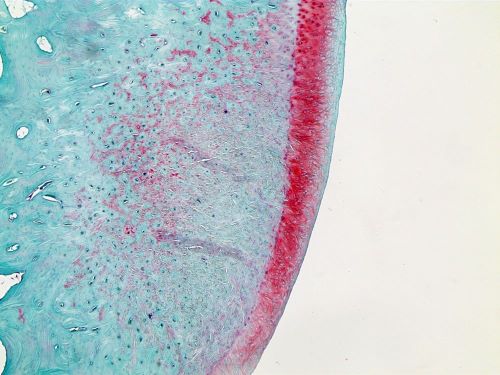
Next, Nguyen and his colleagues analyzed how well the damaged cartilage had healed. They found that rabbits with implanted piezoelectric films that participated in the exercise regimen had the greatest cartilage regeneration; in fact, the entire defect in these damaged joints was filled with new tissues. Computed tomography (CT) analysis revealed that rabbits in this group also had better bone regeneration around the knee joint.
When the researchers evaluated the cartilage tissues under a microscope, they confirmed that animals treated with implanted piezoelectric films and an exercise regimen had the most improved regeneration, compared to the other groups. These animals had abundant chondrocytes (cartilage cells) and collagen protein in the treated joint. “Treatment with the piezoelectric film along with exercise resulted in a beautiful layer of cartilage that resembles the native tissue of our models,” Nguyen said.
While the results are promising, Nguyen stressed that this work is not yet ready to be evaluated in humans. “The materials we use in our films are very safe and the manufacturing process is very scalable, so in that sense our technology is very translational,” he said. “However, important next steps will include evaluation of our films in larger animals, with higher joint loads, before studies in human patients can begin.”
This study was funded by a grant from NIBIB (R21EB024787) and partially by a grant from NIAMS (to the National Institute of Arthritis and Musculoskeletal and Skin Diseases; R21AR078744).
Study reference: Yang Liu, Godwin Dzidotor, Thinh T. Le, Tra Vinikoor, Kristin Morgan, Eli J. Curry, Ritopa Das, Aneesah McClinton, Ellen Eisenberg, Lorraine N. Apuzzo, Khanh TM Tran, Pooja Prasad, Tyler J. Flanagan, Seok-Woo Lee, Ho-Man Kan, Meysam T. Chorsi, Kevin W. H. Lo, Cato T. Laurencin and Thanh D. Nguyen. Exercise-induced piezoelectric stimulation for cartilage regeneration in rabbits. Scientific translational medicine, 2022; 14 (627). DOI: 10.1126/scitranslmed.abi7282