Learning how the brain works is a formidable challenge, helped by the development of innovative brain imaging technologies. Now, scientists at Duke University, supported by funding from the National Institute of Biomedical Imaging and Bioengineering (NIBIB), have developed an ultrafast photoacoustic imaging system capable of visualizing functional and molecular changes in the brain related to major brain disorders.
Photoacoustic microscopy (PAM) is a research and diagnostic tool that fires pulses of laser light at an organ of interest. The pulses cause vibrations that release an ultrasound wave, which is captured to form an image. PAM uses different wavelengths of laser light to target specific structures and even molecules in the body. For example, specific wavelengths of laser light that target hemoglobin can be used to image the array of blood vessels throughout an entire tissue.
Researchers at Duke University are working to develop PAM systems that can be used to study brain functions. The challenge has been to develop a PAM system that images the brain fast enough to track hemoglobin as it moves through the brain’s blood vessels and has a wide field of view that can track blood moving through relatively large brain regions.
The work was led by Junjie Yao, PhD, assistant professor of biomedical engineering and faculty member at the Duke Institute for Brain Sciences. Yao and his collaborators used innovative engineering approaches to develop ultrafast functional photoacoustic microscopy (UFF-PAM), which allowed them to image functioning blood vessels throughout the mouse brain. The new system was used to study blood oxygenation, vascular constriction and dilation, and other hemodynamic measures in mice in response to experimentally induced brain disorders such as stroke.
“The Duke team and their collaborators continue to advance the promising field of photoacoustic imaging to study brain function in disease and health,” explained Randy King, PhD, director of the Ultrasound: Diagnostic and Interventional program at NIBIB. “This work is an important step toward identifying vascular changes associated with important brain disorders, information that could improve treatments for diseases such as stroke, trauma and even dementia.”
A major innovation of UFF-PAM is the use of a polygonal scanner: a disk with 12 facets flattened around the edge. The multifaceted scanner rotates at extremely high speed over the tissue to be examined. The rotating facets direct pulses of laser light into the tissue and collect the returning ultrasound signals, resulting in the fastest PAM imaging to date.
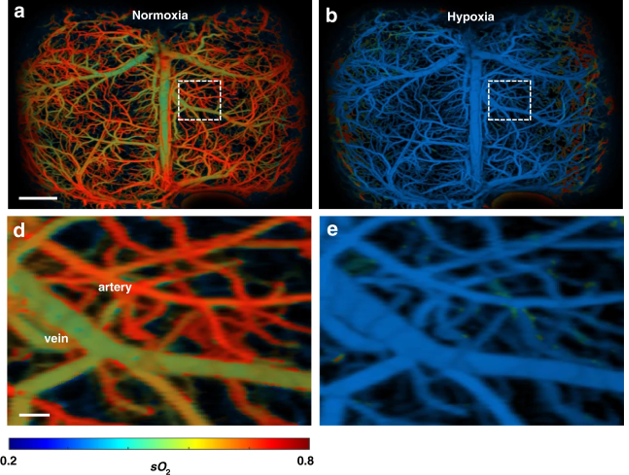
In a proof-of-concept experiment, researchers imaged the brain of a mouse with an induced stroke. UFF-PAM images of blood hemoglobin are color-coded from high (shown in orange-red) to low (shown in green and blue). The stroke caused red-orange oxygenated arteries in the brain to turn blue-green, as expected when the stroke dramatically reduced the oxygen supply to the brain (hypoxia). The procedure is reversible and the return of oxygen to the brain caused the blue-green hypoxic arteries to reoxygenate and return to an orange-red color.
Also in the stroke model, the team, for the first time in PAM, observed a phenomenon not fully understood and of great interest to researchers and clinicians. It is a wave, called a propagated depolarization (SD) wave, that emanates from the central area of the stroke and moves through the brain. In the stroke model, UFF-PAM detected an SD wave that caused vasoconstriction as it moved from the main stroke infarct region through the left hemisphere of the mouse brain. The wave can be seen because vasoconstriction reduces the amount of oxygenated blood in the vessels, causing the arteries and arterioles to darken and then reappear as the wave moves through the brain.
SD waves are of great interest because researchers are not completely sure of their function. “SD waves could be an indication of the level of severity of an injury, making them a potential diagnostic tool,” Yao explained. “The nature of the waves could also offer clues about the type and extent of brain injury, which could inform and optimize treatment.”
The team now uses UFF-PAM to study a number of diseases. For example, UFF-PAM was used to image the mouse placenta in response to an alcohol challenge. Surprisingly, the challenge caused an increase in oxygen in the placenta. A significant finding because the placenta functions to maintain low oxygen concentrations early in gestation, which is necessary for proper development of the placenta and fetus. Their result suggested that fetal abnormalities may be due to a toxic increase in oxygen in response to alcohol.
While the group plans to use UFF-PAM primarily in animal models for basic research, Yao revealed plans for a portable UFF-PAM device for use in humans. One goal of such a device would be to quickly detect sepsis, a serious blood infection. UFF-PAM could be used to monitor blood vessels under the skin for the onset of the deadly condition, allowing for rapid and potentially life-saving treatment.
The study appears in the scientific journal Light: Science and Applications 1. The work was funded through grants R01 EB028143, R21 EB027304, R21EB027981 from the National Institute of Biomedical Imaging and Bioengineering, the National Cancer Institute, the National Institute of Neurological Disorders and Stroke, the National Heart, Lung, and Blood Institute, the from the Chan Zuckerberg Initiative on Deep Tissue Imaging and the Silicon Valley Community Foundation.
-Written by Thomas Johnson, Ph.D.