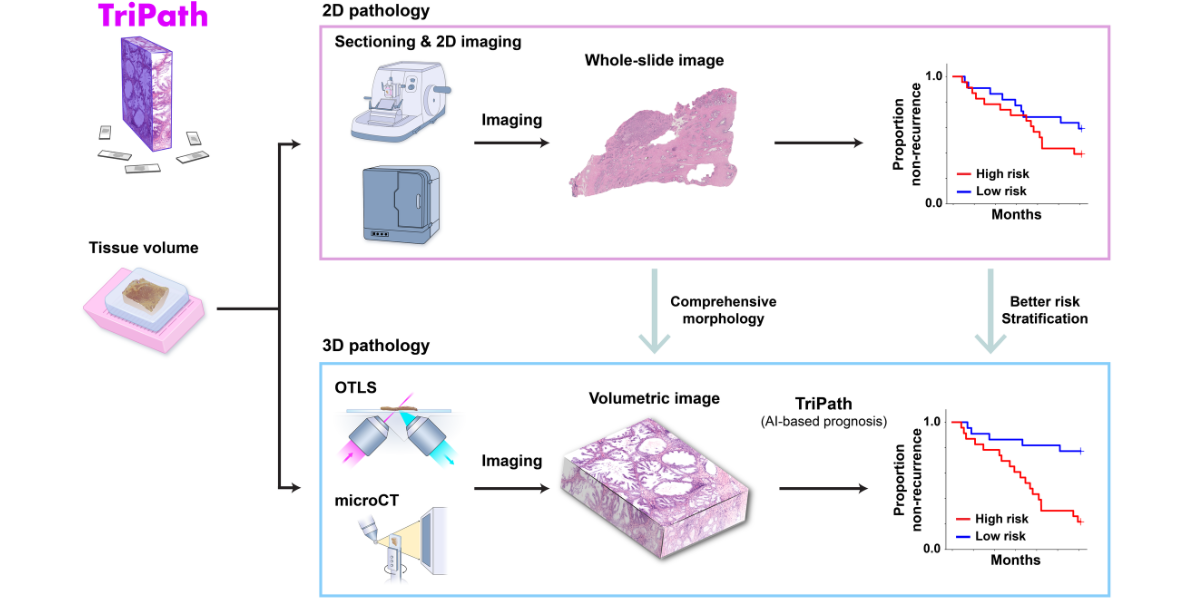
The analysis of human tissue samples—taking thin sections, staining them, mounting them on slides, and viewing them through a microscope—has not fundamentally changed in more than a century. While this technique can help diagnose and predict disease progression, it is inherently limited by its two-dimensional nature. Humans, after all, are not flat, and 2D image slices can only tell us so much about our 3D bodies (and the tissues they contain).
3D pathology techniques have recently emerged, but their use in a clinical setting poses several challenges. In particular, the information captured from high-resolution 3D samples is enormously more complex than that gathered from traditional 2D image slices. Manually analyzing 3D tissue data sets is almost impossible and is likely to miss important details that could help improve prognosis.
A team of NIBIB-funded researchers recently developed TriPath, an artificial intelligence platform that can analyze 3D pathology images to predict disease outcomes. His method, reported in the magazine Cellhad improved performance in predicting prostate cancer outcomes compared to traditional pathology approaches, such as analysis by expert pathologists using 2D images.
“Computational pathology is a growing field and many algorithms are being developed to analyze tissue samples,” said study author Jonathan Liu, Ph.D., professor of mechanical engineering at the University of Washington. “These success stories, based on 2D histological images, suggest that we could further revolutionize the field using 3D pathology data sets that are 100 times larger and that accurately capture native tissue morphology.”
3D tissue sampling and AI analysis
Instead of finely cutting the biopsied tissue and staining it, preparing samples for 3D imaging can be much simpler. Gentle approaches are available to make thick tissues transparent to light while maintaining the delicate structures and molecular constituents of the native tissue. Additionally, larger tissue volumes can be imaged comprehensively, such as whole biopsies, and the sample can be stained with reversible dyes or no dye at all. In this study, the authors used two previously described methods:open light sheet microscopy (OTLS) and microcomputed tomography (microCT)—to obtain 3D images of tissue biopsies and surgical specimens. While these imaging modalities are relatively expensive and are currently only used for research purposes, they can image tissues non-destructively and do not require an experienced histotechnologist to prepare samples, Liu explained.
“Traditionally, pathologists look at several cross-sections per biopsy, which may represent only 1% of the total tissue biopsied,” Liu said. “3D imaging techniques such as OTLS and microCT can provide a more holistic view of the sample, allowing the analysis of more tissue volume and also the discovery of microstructures and cellular interactions that could improve clinical decision making. However, mining these immense data sets to extract information for doctors and patients remains an unmet need so far.”
Liu and his Harvard Medical School colleagues, including Andrew H. Song, Ph.D. and Faisal Mahmood, Ph.D., designed TriPath to predict clinical endpoints from 3D pathology images. This deep learning-based process automatically divides large, data-rich tissue into smaller segments, which are then analyzed individually. Specific predictive characteristics within each segment are identified and weighted, and then information from each segment is combined to provide an overall forecast prediction.
Training for TriPath was “weakly supervised,” which is not to say its predictions are inaccurate. “Instead of using pixel-level annotations provided by expert pathologists, we can train AI models based on ‘tags’ that are applied to the entire sample or the entire patient, such as survival or disease progression over time,” Liu explained. The ‘weak tag’ in prostate cancer used to train TriPath was biochemical recurrenceor an increase in prostate-specific antigen (PSA) in the blood, within five years after surgical removal of the prostate from cancer patients. Biochemical recurrence is a possible indication that the cancer has returned.
“A major challenge in prostate cancer care is risk stratification, since most patients with this disease would never die from it,” Liu said. “If we can accurately identify the small fraction of patients with aggressive cancer who would benefit most from intensive therapies, we could prevent the rest of the patients from receiving unnecessary treatments that can have significant side effects, such as incontinence and impotence.”
Comparison of TriPath to Standard Methods
The ability of TriPath to predict biochemical recurrence was evaluated using two separate groups of prostate cancer samples, one with images collected by OTLS and the other with images collected by microCT. The researchers found that TriPath could predict outcomes using either imaging modality, and that its models were most accurate when TriPath analyzed more tissue volume and when the process incorporated 3D morphological features of the tissue samples.
The researchers also compared TriPath’s performance with that of six experts. genitourinary pathologists from four different countries. TriPath could more accurately predict biochemical recurrence than expert pathologists, both compared to each pathologist individually and to their averaged predictions.
“Our results show that incorporating the native 3D morphology of the volumetric tissue sample can lead to better prognostic predictions, which could ultimately improve patient care,” Liu said.
Looking forward
While analyzing 3D pathology samples is not currently part of routine clinical practice, Liu and his team believe this technology has the potential to augment 2D pathology approaches and improve outcomes. “This work provides initial evidence to support the value of 3D pathology and should stimulate additional, broader studies on computational 3D pathology in the future,” he said.
Future work will require the analysis of additional prostate tissue samples, collected from various locations and using different imaging techniques. The researchers also hope that this methodology can be applied to other cancer types and diagnostic tasks. “We anticipate that TriPath will also evolve to reflect the rapid and impressive advances in the fields of machine learning and computer vision to achieve even better forecasting performance,” said Song, who was the first author of this study.
“The computational process described here has the potential to transform the way we analyze human tissue samples, unlocking invaluable information from everyday biopsies,” said Afrouz Anderson, Ph.D., program director of NIBIB’s Division of Applied Science and Technology. “Further studies will be needed to ensure this technique performs accurately across multiple imaging modalities and in all patient populations.”
This research was supported by grants from NIBIB (R01EB031002), the National Cancer Institute (NCI; R01CA268207 and T32CA251062), the National Institute of General Medical Sciences (NIGMS; R35GM138216), and the Office of the Director (S10OD023519). This study was also supported by a grant from the Department of Defense Prostate Cancer Research Program through W81WH-18-10358 and W81XWH-20-1-0851.
Study reference: Andrew H. Song et al. Analysis of 3D pathology samples using weakly supervised AI, Cell 1872502-2520, 2024. https://doi.org/10.1016/j.cell.2024.03.035