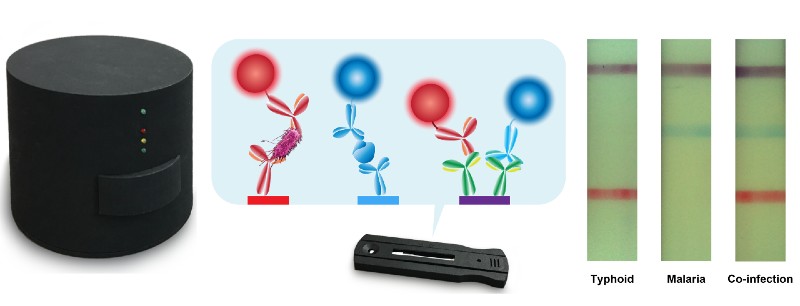
Malaria and typhoid fever are important health problems in tropical regions, but accurate diagnosis is often hampered by overlapping or nonspecific symptoms and limited access to diagnostic means. Now, NIBIB-funded scientists have developed a single point-of-care assay that identifies malaria, typhoid (or both simultaneously) in just 15 minutes.
Every year, worldwide, more than 240 million cases of malaria cause approximately 600,000 deaths; Between 10 and 20 million cases of typhoid fever account for approximately 150,000 deaths. Both are febrile illnesses (cause high fever), which is usually the reason a person visits a clinic. Currently, most tests to determine which disease is present require sophisticated laboratory equipment and trained personnel that are not available in areas such as sub-Saharan Africa, where malaria, typhoid fever and poor health infrastructure coexist.
To address the problem, scientists at Cornell University in Ithaca, New York, have developed a portable test that combines the speed, accuracy and low cost necessary for practical use in low-resource settings where the two diseases are widespread and can even infect the same individual.
The Cornell group is a 10-year collaborative effort that aims to improve public health by creating affordable diagnostics that work at what the team describes as the point of need: anywhere in a community where a rapid diagnosis can improve access to treatment. The collaboration was created by Dr. David Erickson, Sibley College Professor at Cornell’s Sibley School of Mechanical and Aerospace Engineering, and Dr. Saurabh Mehta, Janet and Gordon Lankton Professor of Global Health, Epidemiology, and Nutrition in the Division of Nutritional Sciences.
“The Cornell group specializes in point-of-care (POC) testing that can have a real impact in the developing world,” explained Tiffani Lash, Ph.D., director of the NIBIB program in Point-of-Care Technologies and Diagnostics. “This important test can be added to a growing list of his achievements that include POC tests that detect nutritional deficits, a number of infectious diseases, cholesterol levels and even cancer.”
The new test that can identify whether a patient is infected with malaria, typhoid or both is known as a lateral flow assay. Like pregnancy tests and home COVID-19 tests, a patient’s swabs or liquids are placed on a test strip where they flow across the strip. The appearance of different colored lines indicates whether an infection is present. An important part of such tests is a control line that indicates that the test was performed correctly.
The new assay was tested using human serum “spiked” with malaria and typhoid antigens, as well as patient samples from several individuals. With samples containing antigens from both pathogens, the lateral flow assay produced a red line indicating typhoid, a blue line indicating malaria, and a purple control line. Additional clinical samples are being tested with the new assay to optimize the test’s performance and sensitivity.
A key innovative aspect of the technology is the cleverly designed chemistry that allows simultaneous detection of malaria and typhoid fever on a single test strip using just a few drops of serum, which is described as “duplex” because it can detect two diseases. Another innovation is an inexpensive portable optical reader, which has extremely high sensitivity that can detect lines appearing on test strips that would not be clearly visible to the naked eye. One reader, which could serve the entire clinic, costs about $70, and individual tests cost about $2 each.
“Ultimately, diagnosis is about treating: identifying the right disease to facilitate appropriate treatment and administering it as soon as possible,” Mehta explains. “For example, without an accurate diagnostic test, healthcare workers in low-resource settings often have to resort to presumptive treatment. They may simply treat malaria, if it is more common in the region. However, if the individual has typhoid, the disease may worsen, and time lost in correct typhoid treatment may result in a critical situation.”
Another issue is the problem of the development of resistance to antibiotics. Without diagnostics that allow treatment with the most effective drug for the specific disease, overuse of broader-spectrum antibiotics eventually results in the development of antibiotic resistance, which can leave a population without effective treatments for such diseases.
From an engineering standpoint, Erickson explained the reasoning behind developing the portable reader that scans test strips and displays the results. “We have developed a series of tests that use a reader that connects directly to a smartphone, but in the field people have different smartphones, which makes it impractical to build a single piece of hardware (a reader) that can connect correctly to all smartphones.”
To solve this problem, the team is developing a reader that will be able to communicate with all types of smartphones via Bluetooth. Although the current reader can simply display test results, by incorporating Bluetooth capability, test results can be added to a person’s health record on their smartphone. Collecting such information can also be extremely valuable in tracking the spread of diseases in a population and collecting other relevant information, such as the emergence of disease variants, that can be used to develop more effective public health programs.
Recently, the group has pivoted its engineering and global health expertise to focus on creating POC tests to aid in the diagnosis of conditions affecting maternal and child health, a critical public health issue affecting mothers and children around the world.
The work was published in the journal Analytical Chemistry1 and was supported by grant R01EB021331 from the National Institute of Biomedical Imaging and Bioengineering.