Left to their own devices, bacteria on our teeth or wounded skin can become encased in a slimy shell, becoming what’s called biofilm. These bacteria wreak havoc on our tissues and, being protected from antibiotics by slime, are difficult to expel. A new strategy may offer a simple way to break down dirt and destroy bacteria.
Researchers at the University of Pennsylvania and Stanford University have developed sugar-coated gold nanoparticles that they used to image and destroy biofilms. In a study published in the Clinical Research JournalThe authors demonstrated the diagnostic and therapeutic potential of nanoparticles on the teeth and wounded skin of rats and mice, eliminating biofilms in just one minute and outperforming common antimicrobials.
“With this platform, biofilms can be removed without surgically debriding infections, which may be necessary when antibiotics are used. Additionally, this method could treat patients if they are allergic to antibiotics or infected by drug-resistant strains,” said Luisa Russell, Ph.D., program director in NIBIB’s Science and Technology Discovery Division. “The fact that this method does not contain antibiotics is a big advantage.”
Oral biofilms, also known as plaques, formed by bacteria such as mutans streptococcus can cause significant tooth decay. Wound infections, which are commonly caused by Staphylococcus bacteria, can greatly delay the healing process. In either case, the dense network of proteins and carbohydrates within biofilms can prevent antibiotics from reaching microbes throughout the affected area.
But that’s not the extent of the problem posed by biofilms. Not only are they difficult to remove, but they are difficult to discern in the first place.
This new research identified a solution to end both problems with one shot: gold.
Gold is non-toxic and easily converts energy from light sources into heat, making it an ideal candidate for photothermal therapy, a strategy that uses the heat from nanoparticles to kill nearby pathogens. In addition to generating heat, the nanoparticles emit detectable ultrasound waves in response to light, meaning gold particles can be visualized using a technique called photoacoustic imaging.
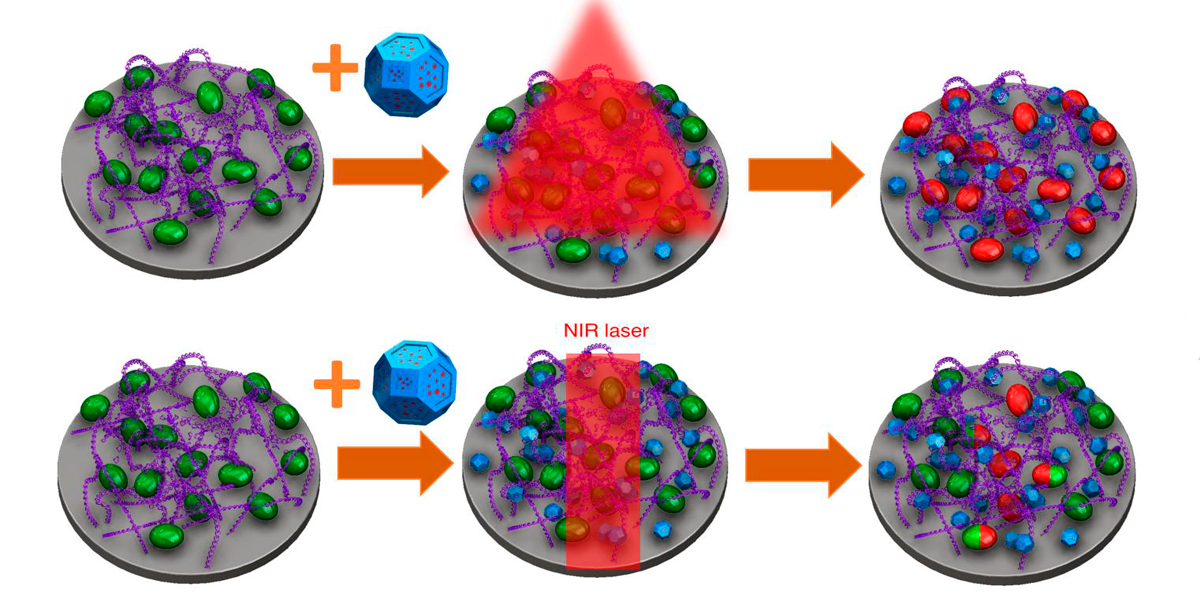
In the new study, the authors encapsulated gold spheres within larger cage-shaped gold nanoparticles to optimize their response to light for imaging and therapeutic purposes. To make the particles attractive to bacteria, they coated them with dextran, a carbohydrate that is a common component of biofilms.
The researchers evaluated their strategy by applying gold nanoparticles on top S. mutans-infected teeth from ex vivo rat jaws.
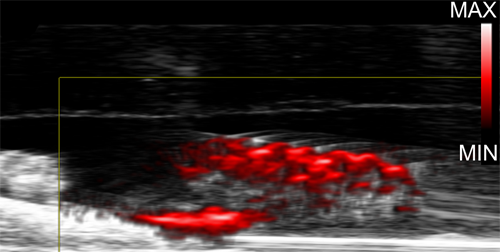
In a photoacoustic imaging test on the teeth, the nanoparticles emitted strong, clear signals, allowing the team to see precisely where the biofilms had absorbed the dextran-coated particles on the teeth.
Then, to evaluate the therapeutic effect of the particles, they irradiated the teeth with a laser. For comparison, they treated other samples of infected teeth with the topical antiseptic. chlorhexidine.
The team observed a stark contrast in the results of the two treatments: photothermal therapy was almost 100% effective in killing biofilms, while chlorhexidine did not significantly decrease the viability of the bacteria.
“The treatment method is especially rapid for oral infection. We apply the laser for one minute, but actually in about 30 seconds we kill basically all the bacteria,” said the study’s first author, Maryam Hajfathalian, Ph.D., a professor of biomedical engineering at the New Jersey Institute of Technology, who conducted this study while she was a postdoctoral researcher at the University of Pennsylvania and Stanford University.
Evaluations carried out in mice with open skin wounds, infected with Staphylococcus aureusThey had similar success, as the heat generated by the nanoparticles far exceeded another antimicrobial agent called gentamicin. Here, the researchers also measured and observed a localized 20°C temperature rise in the biofilm, without causing any apparent damage to the surrounding tissue.
The authors indicate that with more tests they aim to demonstrate whether the strategy can prevent cavities or accelerate healing.
“I think it’s important to see how cheap, simple and fast this process is. Since we have limited use of antibiotics, we need novel treatments like this as a replacement,” Hajfathalian said.
This research was supported by grants from the NIH, including NIBIB (K99EB028838), the National Institute of Dental and Craniofacial Research (R01DE025848), the National Heart, Lung, and Blood Institute (R01HL131557), and the National Institute of Allergy and Infectious Diseases (T32AI007502).
This prominent scientist describes a basic research finding. Basic research increases our understanding of human behavior and biology, which is critical to promoting new and better ways to prevent, diagnose, and treat diseases. Science is an unpredictable and incremental process: each research advance builds on past discoveries, often in unexpected ways. Most clinical advances would not be possible without knowledge of fundamental basic research.
Study reference: Maryam Hajfathalian et al. Theranostic gold-on-gold cage nanoparticles enable photothermal ablation and photoacoustic imaging in biofilm-associated infection models. Clinical Research Journal. DOI: 10.1172/JCI168485
About the graphics: The graphics are adapted from figures under the Creative Commons Attribution 4.0 International License.