Early detection of infectious diseases can be key to successful treatment, but we often don’t know we are infected until symptoms appear. What if our bodies could detect the presence of an infectious pathogen before the onset of illness?
Synthetic biology engineers are finding ways to do just that.
Synthetic biology is the design and construction of new biological parts and systems, and the redesign of existing and natural biological systems for specific purposes.
With an eye toward early disease detection, synthetic biology engineers at the University of Wisconsin have engineered bacteria that find and detect DNA fragments shed from infectious pathogens. Because the DNA of a pathogen in an individual could be identified before the onset of symptoms, the system offers the promise of extremely early detection of life-threatening diseases such as sepsis, where rapid detection is critical for successful treatment.
The work was performed in the laboratory of Ophelia Venturelli, Ph.D., assistant professor of biochemistry, bacteriology, and chemical and biological engineering. The laboratory specializes in the development of microbial control systems to address problems in medicine, agriculture and the environment. An important area of research is how the human microbiome affects health and disease and how it can be modified to treat a variety of disorders. The work was co-led by postdoctoral fellow Yu-Yu Cheng and graduate student Zhengi Chen.
To design their bacterial DNA sensor, Venturelli’s team took advantage of the natural ability of a common bacteria bacillus subtilis (B. subtilis) to capture DNA from its environment.
A series of genes were integrated into the B. subtilis genome. This “genetic program” includes a region with a specific DNA sequence that matches the target organism. Researchers call this region a “cassette” because it is designed to be easily swapped (in and out) with DNA sequences from the various organisms targeted for identification. The cassette region is part of a genetic program that is activated when B. subtilis It finds the target sequence and delivers it to the cell. Upon activation, the program directs a cascade of events that culminates in a fluorescent signal indicating that the bacterial sensor has detected the target organism.
In one of the first experiments, the team inserted a piece of DNA from the bacteria. Escherichia coli in the cassette region and tested the ability of the sensor bacteria to detect Escherichia coli The DNA was mixed with the solution. when he Escherichia coli DNA was introduced into the cell and found its identical material. Escherichia coli DNA on “cassette”. Specialized bacterial proteins then swapped the two sequences, activating the genetic program. The genetic program directs the production of green fluorescent protein (GFP), which created a strong fluorescent signal indicating that Escherichia coli DNA was present in the sample.
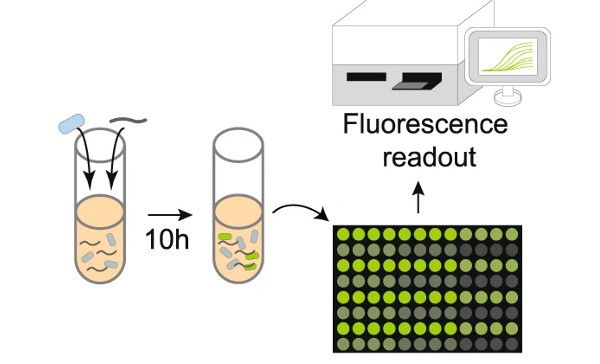
Having demonstrated that its DNA sensor was capable of detecting the Escherichia coli DNA, the Wisconsin team went on to test the system’s ability to identify human pathogens. Later experiments showed that the B. subtilis The DNA sensor could be programmed to successfully detect a variety of human bacterial pathogens. The human intestinal pathogen, Salmonella typhimurium (S. typhimurium), and the pathogen of human skin and respiratory tract. Staphylococcus aureus (S. aureus) were among those that were successfully detected by the B. subtilis DNA sensor.
“The ability of these sensors to detect human pathogens opens the possibility of a number of innovative diagnostic and therapeutic approaches,” explained Jermont Chen, Ph.D., program director at the National Institute of Biomedical Imaging and Bioengineering, which funded the study. “For example, the system could be used to rapidly diagnose and treat infectious diseases with specific antibiotics, rather than broad-spectrum antibiotics. This would be particularly valuable for high-risk infections such as sepsis, where the time spent waiting for bacterial culture results quickly decreases the likelihood of successful treatment.”
The lab is particularly interested in the microbiome: the millions of bacteria that reside in the human gut and throughout the body and interact with human cells in health and disease.
Venturelli explained the potential use of the sensor in the human microbiome. “This work opens up the possibility of designing sensors that live harmlessly in the human body’s ecosystem and act as disease sentinels. The appearance of DNA from harmful bacteria growing in the intestine, for example, could be detected by the B. subtilis sensor that resides there, sending an early signal of a developing disease that allows for early treatment.”
Having successfully designed the DNA sensor, the team is now focusing on the improvements needed to advance the technology towards a practical application. These include increasing efficiency B. subtilis absorbs DNA in the surrounding environment and increases the efficiency of detecting pathogenic DNA at low concentrations. Longer-term goals include developing more complex genetic programs that allow sensitive bacteria to identify a pathogen while producing the therapeutic proteins needed to immediately combat an infection.
The study was published in the journal Nature Communications.1. The work was supported by grant R01EB030340 from the National Institute of Biomedical Imaging and Bioengineering and grant HR0011-19-2-0002 from the Defense Advanced Research Projects Agency (DARPA).
–Written by Thomas Johnson, Ph.D.