The introduction of medical devices (commonly made from materials such as titanium, silicone or collagen) into our bodies can trigger a number of different immune responses. While some responses can harm our body, others can help heal it. Researchers haven’t fully understood the reason behind the body’s reactions, but a new study completes a critical piece of the puzzle.
By closely examining the immune system’s polarized responses to two different materials implanted in mice, researchers at the National Institute of Biomedical Imaging and Bioengineering (NIBIB) have identified key factors for implant-induced regeneration and damage. The authors suggest that their findings, published today in Nature materialsThey help lay the foundation for the design of new medical devices that influence the immune system to help, rather than harm, the body.
Medical devices such as pacemakers, breast implants, or knee replacements can trigger hostile immune responses that can damage not only the implants themselves, but also the surrounding tissue. The clinical solution has been to administer drugs that inhibit the body’s immune response, known as immunosuppressants, which are not always effective and carry serious risks.
Due to complications including immune-mediated damage, additional surgeries are almost always required, either months or years later, to remove or replace implants that have become ineffective or dangerous.
However, some implants, such as naturally occurring tissue grafts, have been shown to direct the immune system in a more desirable direction, stimulating reparative processes. While natural biomaterials are not suitable for all medical applications, they may still hold valuable lessons for other types of devices.
“If we could figure out what aspect of these different materials causes certain immune responses, we would have more control in designing materials and devices that encourage the responses we want,” said lead author Kaitlyn Sadtler, Ph.D., Earl Stadtman Tenure-Track Investigator and head of NIBIB’s Immunoengineering Section.
To find explanations for the different reactions to the different devices, the authors of the new study removed volumes of muscle from the paws of mice and filled the gaps with two biomaterials known to cause opposite reactions.
One of them was the decellularized submucosa of the small intestine (porcine tissue that has been stripped of its cells, leaving mainly collagen) that stimulates reparative immune processes and has shown promise as a clinical solution for abdominal injuries. The other was polyethylene, a plastic used to make orthopedic implants that can cause long-term inflammation and scarring when small pieces of it break away from the surrounding tissue. A third group of injured mice received no treatment for comparison.
Using a process called flow cytometry, Sadtler and his co-authors identified and counted several cell types from tissue recovered from the mice.
As expected, samples implanted with submucosa showed patterns more closely associated with anti-inflammation and repair than those implanted with polyethylene. But, of the many differences between the samples implanted with the two types of materials, the amount of one type of cell present was particularly surprising.
The submucosa samples contained much more of a specific cell type called cDC1, which belongs to a class known as dendritic cellsThey help the immune system distinguish between friends and enemies.
What’s more, when researchers used mice genetically altered to hinder the development of the dendritic cell subtype, inflammation and scarring were rampant and new muscle growth was impaired, regardless of the implanted material.
“These dendritic cells likely play a key role in calming the immune system. So when we saw that they were enriched in the samples with porcine submucosa, we wanted to dig deeper to understand how and why these cells were at the injury site,” Sadtler said.
Through a series of experiments, the researchers determined that a type of immune cell called natural killer cells secreted chemical signals to attract the dendritic cell subtype, a well-documented process observed in immune responses to tumors.
In the context of cancer, natural killer cells are activated by molecular danger signals released by injured or dying cells. These signals would likely also be present in the pigs’ submucosa, but not in the polyethylene, making these molecules a likely driver for the drastic difference in how the two groups of animals responded, Sadtler explained.
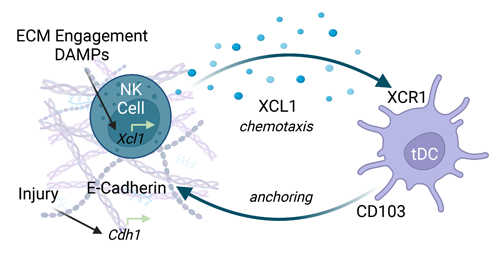
After identifying some preliminary mechanisms by which the material induces this cellular recruitment, Sadtler’s team plans to identify the specific proteins and molecules that initiated the reparative responses, with the goal of harnessing those molecules to dampen autoimmunity and amplify regenerative processes.
If their strategies bear fruit, then they could be useful for modifying existing devices (adding protein-filled coatings to the surface of a pacemaker, for example) or informing the design of entirely new devices. By playing right with the body’s complex immune system, medical devices of the future could be safer and last much longer.
“Implants often cause pain and, in many cases, need to be removed within a few years. We are seeking to eliminate that pain by extending the life of implanted medical devices to the lifespan of patients,” Sadtler said.
This research was funded by the NIH Intramural Research Program.
This Science Highlight describes a basic research finding. Basic research increases our understanding of human behavior and biology, which is critical to promoting new and better ways to prevent, diagnose, and treat diseases. Science is an unpredictable and incremental process: each research advance builds on past discoveries, often in unexpected ways. Most clinical advances would not be possible without knowledge of fundamental basic research.
Study reference: Lokwani, Josyula, Ngo et al. Proregenerative biomaterials recruit immunoregulatory dendritic cells after traumatic injury. Nature materials (2023). DOI: 10.1038/s41563-023-01689-9