Gene editing therapies(techniques that modify DNA to treat or prevent diseases) have the potential to transform the field of drug development. By making precise edits to the genome, problematic genes could be modified or deleted, representing durable therapies for genetic disorders that currently have no treatment.
There are currently few gene editing therapies. Last year, the FDA approved a gene editing therapy for sickle cell anemia, a landmark treatment that uses CRISPR to modify blood stem cells. But like most experimental gene-editing treatments, this therapy requires a stem cell transplant, an expensive and time-consuming procedure that involves extracting stem cells from a patient, modifying them to correct the disease-causing defects, and reintroducing them to the patient. Finding a way to modify a patient’s stem cells live—without having to remove them from the body—could revolutionize the field of genome editing.
UT Southwestern researchers are working to bring live gene editing in the foreground. By rationally engineering lipid nanoparticles, this collaborative team developed a way to effectively target specific organs in the body to precisely deliver a therapeutic payload, including gene-editing molecules. Their research showed that a single treatment with their nanoparticles resulted in long-lasting gene editing in the lungs of mice for almost two years. Furthermore, their technique showed promise in correcting a mutation present in a currently untreatable form of cystic fibrosis in various models of the disease. The research was recently published in Science.
“There is a real desire to imagine a single injection of a drug that can correct disease-causing and disease-causing mutations,” said the study’s senior author, Daniel Siegwart, Ph.D., a professor at UT Southwestern Medical Center. “Our preclinical platform illustrates a potential method to achieve long-term gene editing in the lungs, representing a new treatment approach for a variety of genetic respiratory conditions.”
Ordering it
Lipid nanoparticles, a popular drug delivery method thanks to the success of COVID-19 mRNA vaccines, are typically composed of four different lipids (fats) that envelope their therapeutic payload. While these traditional lipid nanoparticles are effective at transporting and protecting their payload, when infused into a patient, they accumulate in a specific organ: the liver.
“Traditional lipid nanoparticles are remarkably similar to low-density lipoproteins (LDL) in terms of size and chemical composition,” Siegwart explained. “LDL particles naturally travel to the liver to be broken down, so it makes sense that lipid nanoparticles would accumulate there as well.”
To allow delivery to tissues other than the liver, Siegwart and colleagues previously developed a new class of particles, called selectively organ-targeted nanoparticles (SORT). In addition to the four conventional lipids found in traditional lipid nanoparticles, SORT nanoparticles contain a fifth lipid that targets the particles to a specific organ. This fifth lipid affects the physicochemical properties of the nanoparticle and attracts different plasma proteins to its surface, two factors that influence absorption by different types of tissues in the body.
“We knew we needed to break the rules of traditional lipid nanoparticle formulations to target tissues other than the liver,” Siegwart said. “Using CLASSIFY, we have shown that we can target nanoparticles specifically to the liver, spleen and lungs of mice. “Whether this technique could lead to durable, tissue-specific gene editing remained an open question.”
Long-term lung edition
Getting the lipid nanoparticles to the right organ is an important first step. But for effective gene editing, it’s also important to target the right type of cell—specifically, stem cells and progenitor cells, or cells that can become different types of cells.
“Rodent lung cells are estimated to be renewed and regenerated approximately every few months,” Siegwart said. “If genome correction is achieved in mature cells, the effects will be temporary: as soon as the new cells are born, they will no longer have those events corrected and defective genes will be produced again.”
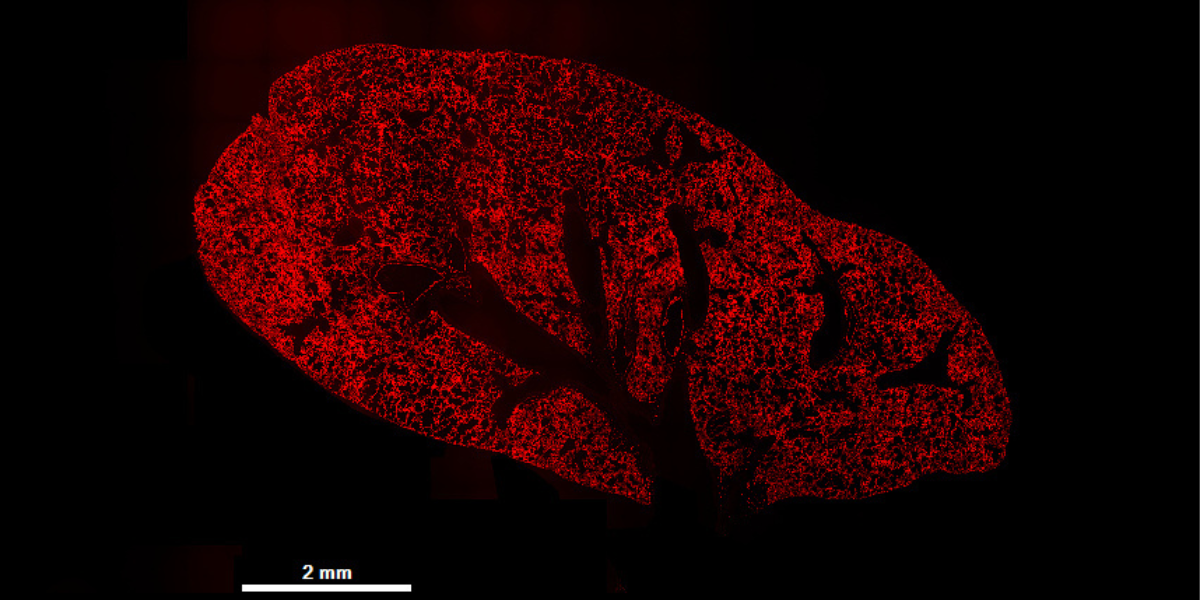
To understand whether their SORT nanoparticles could achieve long-lasting genome editing in the lungs, Siegwart and his colleagues used a genetically modified mouse that has the ability to produce a red fluorescent protein, but only if its genome is edited in a specific way. “It’s a beautiful model because it allows us to quantify which specific cell types have been edited by the nanoparticles, and we can easily visualize where these nanoparticles are effective,” he said.
The researchers administered SORT nanoparticles filled with gene-editing molecules to mice and then evaluated lung tissues at ten different time points, from two days to 22 months after the injections. They found that the red fluorescence was evenly distributed throughout the lungs at each time point. Furthermore, cell-specific analyzes revealed that genome editing was achieved in multiple different cell types and that editing was maintained over the course of the study, almost two years after treatment with the nanoparticles.
“When we tracked the animals over time, we found that these genome editing events were completely persistent, and almost two years later, the animals were edited in the same way as on the second day,” Siegwart said. “This indicates that nanoparticles can successfully edit stem and progenitor cell populations that then differentiate over time into healthy, edited cells.”
Evaluation in cystic fibrosis models.
With a method to achieve long-term gene editing in hand, Siegwart and his colleagues turned their attention to a genetic lung disease: cystic fibrosis.
Cystic fibrosis is caused by mutations in a chloride pump, a protein that can regulate the concentration of salt inside and outside the cell, Siegwart explained. “When this protein does not function properly, there is an improper salt balance, which causes thick, sticky mucus to build up in the lungs. This can lead to a host of different breathing problems, infections, and eventually persistent lung damage that can lead to the need for a lung transplant.”
Approximately 90 percent of patients with cystic fibrosis can be treated with a innovative medicine That helps this chloride pump work more efficiently. However, some patients have mutations in their genome that lead to a truncated, non-functional form of this protein, or the protein is not produced at all. These patients currently have no approved treatment options.
In this context, Siegwart and his colleagues focused on one of these “non-drug” cystic fibrosis mutations. They packed their lung SORT nanoparticles with a gene editor that corrects the mutation, converting the truncated chloride pump into the normal version of the protein. They then evaluated how well their system performed in cystic fibrosis models.
In experiments using patient-derived cystic fibrosis lung cells, the researchers found that pulmonary SORT could correct the defective gene, effectively restoring chloride pump function by more than 50%. What’s more, in a mouse model carrying this non-drug human cystic fibrosis mutation, the researchers found that lung SORT could achieve genetic correction in nearly 50% of lung stem cells.
“Our early results suggest that this technique could one day correct dysfunctional proteins in the lungs, which would be absolutely transformative in the daily lives of cystic fibrosis patients,” Siegwart said.
Jermont Chen, Ph.D., program director of NIBIB’s Discovery Science and Technology Division, agreed: “Through clever modifications of standard lipid nanoparticles, this team has laid the foundation for a live gene editing platform in the lungs, which could potentially be translated to other tissues in the future. “While future work in relevant animal models will need to be done before this technique can be evaluated in humans, the method described here has the potential to lead to durable treatments for patients with genetic diseases.”
Note: Siegwart is co-founder of the company ReCode Therapeutics, a partner in this study.
This study was funded in part by a NIBIB grant (R01EB025192).
This prominent scientist describes a basic research finding. Basic research increases our understanding of human behavior and biology, which is critical to promoting new and better ways to prevent, diagnose, and treat diseases. Science is an unpredictable and incremental process: each research advance builds on past discoveries, often in unexpected ways. Most clinical advances would not be possible without knowledge of fundamental basic research.
Study reference: Yehui Sun et al., In vivo editing of lung stem cells for long-lasting gene correction in mice. Science 384,1196-1202(2024). DOI:10.1126/science.adk9428