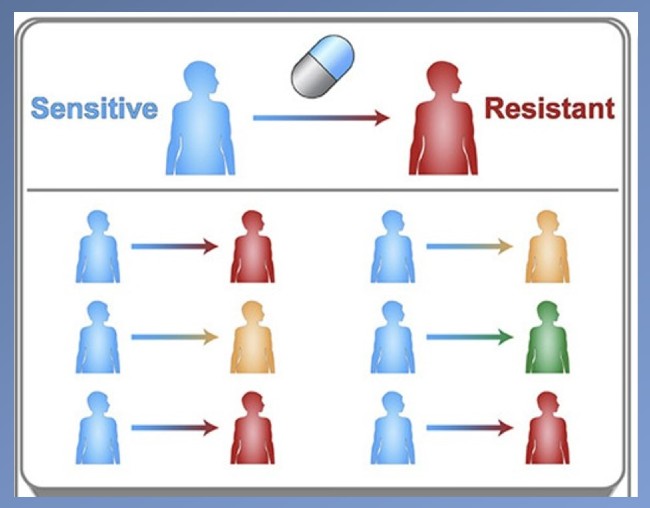
To counteract drug resistance, scientists design new drugs that “adapt” to new mutations and thus kill the cancer cell or pathogen. Now, a NIBIB-funded team of Penn State engineers has a new approach to predicting which mutation has spread the most in a population and should be the target for designing the most effective new drug.
The need to design new drugs that overcome resistance is crucial to combat a mutated virus in a population of people or a mutated protein that causes drug resistance in a population of tumor cells.
“Traditionally, drug developers focus on the most resistant mutation, the one that completely negates the effect of a drug, assuming that it will be the most common,” explained Justin Pritchard, Ph.D., assistant professor of biomedical engineering and holder of the Dorothy Foehr Huck and J. Lloyd Huck Early Career Business Chair.
However, computer models by Pritchard and his team have revealed that mutations that have weaker resistance to the drug can also spread to be the most prevalent in a population of cells. In that case, it would be necessary to select the mutant that is most prevalent, even if it is least resistant, to have the greatest effect in treating the disease.
The group tested their approach on mutant cancer cells. In a study of mutations that emerged in leukemia patients before and after drug treatment, they found that the mutation that most strongly blocked the effect of the original leukemia drug was not the most common. Instead, a less resistant mutant had expanded to become the most prevalent in tumor cells. In that case, a new drug targeting the most moderately resistant mutant would be the most effective at attacking the majority of leukemic cells.
“Similar proteins can be produced using slightly different genetic codes,” Pritchard explained. “Our model found that slight differences caused certain versions of the resistance proteins to be produced more efficiently by the cellular machinery. In the case of leukemia, that type of bias caused the least resistant protein to be the most widespread in the leukemic cell population.” And so, although it has a weaker effect, its ubiquity among the population made it more dangerous than the more resistant but less prevalent mutant.
“The leukemia result demonstrated that mutation bias will drive prevalence when drug resistance does not,” Pritchard said, “and verifies that this modeling approach could be extremely useful in designing a drug that would have the best effect in leukemia patients.” The team also found similar results in breast, prostate and stomach cancer, although the effect was not as striking as in the leukemia analysis.
Researchers continue to refine their model with the goal of incorporating this type of information into the rational drug design process. The approach could significantly improve the current challenge of developing drugs that combat resistant cancers, as well as resistant bacterial and viral infections that occur when diseases spread throughout the population.
This work appeared in the March issue of Cell Reports.1 and supported by an R21 from the National Institute of Biomedical Imaging and Bioengineering NIBIB (R21EB026617).