Researchers at the University of Wisconsin (UW) are adapting a safer, minimally invasive approach to electrically treat pain directly at the source as part of the Long-term initiative to help end addictionYEor the NIH HEAL InitiativeYE. It is a preview of one of the first grants from the National Institute of Biomedical Imaging and Bioengineering (NIBIB) under the NIH HEAL Initiative.YElaunched in 2018 to find solutions to the opioid crisis.
Neuromodulation therapies apply electrical stimulation to the nerves to treat conditions such as chronic low back pain, paralysis, incontinence, migraines, sleep apnea, and obesity. The UW technology was developed by startup Neuronoff, Inc., using primarily private funding and funding from the Defense Advanced Research Projects Agency (DARPA), and the injection of support from the NIH will prepare the device for its first human clinical trials.
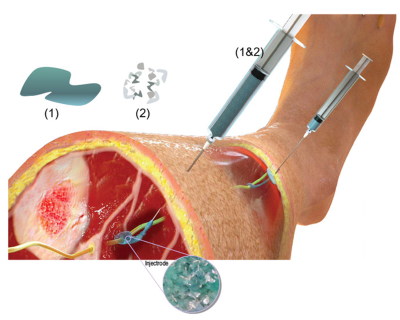
The key innovation is a new type of electrode that can make neuromodulation therapies less invasive, less expensive, less painful, more reliable and much easier to scale to larger numbers of patients.
Currently, the most effective neuromodulation treatments require complex surgical procedures to implant a complex device that is invasive and expensive, making it a treatment of last resort. In contrast, non-invasive neuromodulation devices apply stimulation through electrodes that are placed on the surface of the skin and use a specially designed electrical waveform to block painful signals in the nerves. These electrical waveforms travel through superficial tissues, muscles and nerves to achieve a therapeutic effect. Unfortunately, surface electrodes can cause uncomfortable sensations due to unwanted stimulation of the superficial nerves. This approach has limited effectiveness for the targeted nerves that are deeply buried under the skin.
“To address these limitations, the Wisconsin team has created a new technique that has the effectiveness of an implantable device and is only slightly more invasive than surface electrodes,” said Michael Wolfson, Ph.D., director of the NIBIB Program in Bionic and Robotic Systems. By using an injectable liquid polymer that heals (like household epoxy) next to or around the target nerve, researchers say the device provides a localized stimulus that avoids the side effects of other types of neuromodulation therapy. The new liquid polymer electrode called “Injectrode” is made of softer materials than traditional implantable devices and is mechanically similar to human tissue, allowing it to integrate more easily, while providing electrical conductivity similar to a metal wire.
The UW-Madison team, led by Kip Ludwig, professor of biomedical engineering and neurological surgery, presented their work in Advanced Healthcare Materials, demonstrating that the Injectrode could form flexible structures around a variety of pig nerves and provide an electrical stimulus to a pig’s vagus nerve. The vagus nerve serves as a critical communication link between the brain stem and the rest of the body, particularly the organs and tissues. Vagus nerve stimulation therapy has also been used to treat depression and epilepsy. The group reported that its technology had passed several preclinical safety standards recognized by the Food and Drug Administration for this type of device.
“This is an innovative approach to interacting with the nervous system, and I’m excited to see their HEAL grant push strongly toward first-in-human demonstrations,” Wolfson said. “Even in the midst of a pandemic, the opioid crisis continues unabated and this new technology offers hope to people suffering from debilitating back pain.”
Collaborators at the University of Michigan are developing computer simulations to optimize current delivery to a group of neurons in the spinal nerve called the dorsal root ganglia. This group of nerves is a prime target for neuromodulation therapies. Collaborators at Case Western Reserve University are characterizing and optimizing the mechanical properties of the Injectrode. Finally, what brings it all together for Ludwig’s team is the preclinical work being done by researchers at the University of Pittsburgh who are conducting efficacy testing to aid in the translational process of bringing this pain treatment to market. Neuronoff, Inc. and the Ludwig team will continue to develop the Injectrode with further safety and effectiveness experiments, as well as techniques to reliably administer it and power it with a wireless stimulator.
The work was supported by the NIBIB (1U18EB029251‐01). This work was also supported by the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) Targeted Neuroplasticity Training Program under the auspices of Doug Weber and Tristan McClure-Begley through the Space and Naval Warfare Systems Command (SPAWAR) Systems Center under Pacific Grant (SSC) no. N66001-17-2-4010 and the Grainger Foundation.
James K. Trevathan, et al., “An injectable neural stimulation electrode made from a polymer/metal composite that heals in the body,” Advanced Healthcare Materials, 2019.