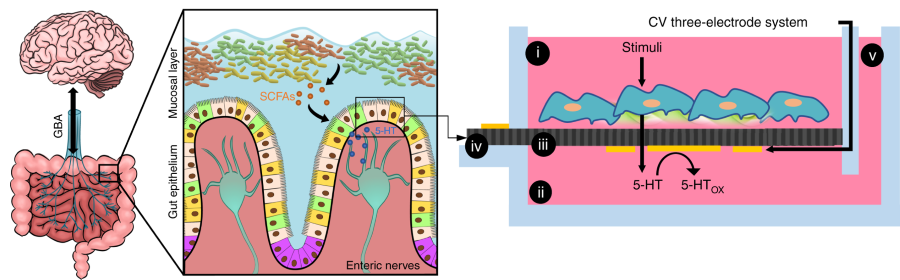
Research into what is known as the gut-brain axis continues to reveal how the brain and gut mutually influence health and well-being. Now researchers are striving to learn more about gut-brain discourse using a model system built in a laboratory dish.
It has been known for decades that the brain and gut, which includes the stomach and intestines, have a relationship that is based on open lines of communication. Scientific studies have shown that gut-brain signaling controls basic functions, such as a full stomach that tells the brain to stop eating, and has been implicated in the development of complex conditions including depression and autoimmune diseases.
And then there are our own conscious experiences that include “trusting our gut,” when faced with a difficult decision, getting dizzy watching the shower scene at home. Psychopathor feeling butterflies when a certain person walks into the room.
Now, researchers at the University of Maryland at College Park have designed an experimental gut-brain system in a lab dish (often called a lab-on-a-chip) to begin identifying the molecules and signaling pathways by which these separate but interdependent organ systems communicate.
“This is impressive tissue, chemical, and electrical engineering,” explained David Rampulla, Ph.D., director of the Synthetic Biology program at the National Institute of Biomedical Imaging and Bioengineering (NIBIB). “The team designed a chip that could support multiple tissue types while integrating chemical and electrical sensors capable of reliably picking up subtle signals taking place between tissues in real time.”
The final chip design features what the team describes as a Transwell system. It has a separate compartment for the “mini-gut” formed by endothelial cells that form the model of the intestinal lining, and a separate compartment for the “mini-brain”, a model nervous system formed by the dissected abdominal nerve cord of a crayfish. The crayfish nerve was used because the crayfish has been a basic animal model for the study of gut-brain axis signaling. A fluid connection between the two compartments allowed the movement and tracking of signaling molecules.
After designing and building their gut-brain model, the research team performed initial tests. One of the central signaling molecules known to play a key role in gut-brain signaling is the neurotransmitter serotonin. The team injected serotonin into the upper part of the intestinal module. The system’s sensors indicated that the neurotransmitter was successfully transferred across the surface of the endothelial cells to the base of the endothelium, where serotonin is naturally released in the intestine.
Within milliseconds, electrical sensors detected the activation of neurons in the crayfish nerve, indicating that serotonin had rapidly diffused into the nerve module, closely reproducing the natural electrophysiological responses observed in animal studies using the crayfish model.
The team is confident that their system will allow real-time monitoring of signaling between both tissues of the gut-brain axis simultaneously for the first time without the need to perform invasive procedures in humans or animals.
Future studies planned for the system include examining how electrical signals from the crayfish nerve cord trigger changes in endothelial cells that are associated with endothelial dysfunction that results in disease. For example, in autoimmune diseases such as irritable bowel syndrome, thinning of the intestinal endothelium occurs, causing endothelial dysfunction and inflammation. Studies in the new system could be extremely valuable in identifying neurochemical signaling involved in disease development and guiding new treatment approaches for such complex diseases.
The work was published in the journal Microsystems & Nanoengineering.1 and supported through grants from the National Science Foundation, the National Institute of Biomedical Imaging and Bioengineering, and the University of Maryland Brain and Behavior Initiative (BBI) Seed Grant Program.
2. Open Access: http://creativecommons.org/licenses/by/4.0/.