Monoclonal antibody treatments offer significant benefits for those suffering from a variety of autoimmune disorders, but these biologics often come with side effects. Now, engineers are developing nanofiber-based treatments that stimulate the body to mount its own biological attack against immune disorders.
Biological treatments for autoimmune diseases consist of monoclonal antibodies that seek out and destroy factors in the immune system that mistakenly mount immune attacks against the body and cause serious, often chronic disorders such as Crohn’s disease, rheumatoid arthritis, and psoriasis.
An immune factor that is commonly overproduced in such diseases is tumor necrosis factor (TNF). Thus, biological treatments consist of injections of monoclonal antibodies that bind to the body and eliminate excess TNF. Unfortunately, in addition to side effects, monoclonal antibody treatments can have varying degrees of effectiveness in different people. Finally, the monoclonal antibodies that attack TNF are finally recognized by the immune system, which destroys them, making subsequent treatments ineffective. This is particularly problematic when the disease is chronic and requires repeated treatments.
Bioengineers at Duke University’s Pratt School of Engineering set out to develop a new approach that has the advantages of consistent TNF neutralization, without side effects. Joel Collier, Ph.D., professor of Biomedical Engineering, and his graduate student, Kelly Hainline, conducted the study, which was funded by the National Institute of Biomedical Imaging and Bioengineering (NIBIB).
The strategy involved building nanostructures to which they could chemically attach protein subunits that could stimulate the type of biological response needed to sequester the excess TNF that causes disease.
“With an estimated 24 million Americans suffering from autoimmune diseases, there is a great clinical need to find effective treatments for these disorders that are less expensive and more effective than existing treatments,” said David Rampulla, PhD, director of NIBIB’s Division of Science and Technology Discovery. “Nanobiology is a promising approach to persuade the body to generate its own immune response to combat these complex diseases. This work in mice is an elegant demonstration of potential therapies using cleverly designed and synthesized nanofibers.”
Duke engineers used a chemical process called supramolecular self-assembly. It sounds complicated but it simplifies things. The technique is used to make many copies of complex molecules with many different parts, each of which has a different (supramolecular) function. The chemistry is cleverly designed so that the added pieces find each other and come together spontaneously (self-assembly).
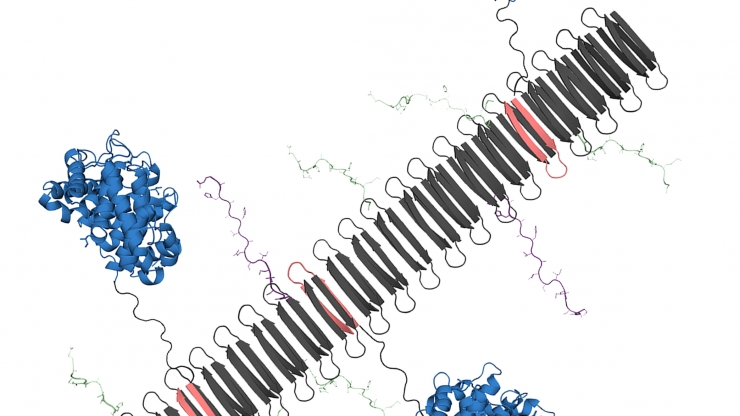
They made a nanostructure, called a nanofiber, with a central backbone of repeating units of a protein. That backbone was used to add the parts that interact with the immune system – the commercial purposes of the supermolecule. One of the main parts of the business is a part of TNF. The other commercial part was a fragment of a protein that stimulates the immune system, called C3dg.
When they put the nanofiber in mice with psoriasis, it worked as well as standard monoclonal antibody treatment. The nanofiber with the TNF pieces caused the mouse’s immune system to produce antibodies against TNF. They found that adding C3dg improved treatment because C3dg increases B cell responses, so more TNF-neutralizing antibodies are produced. Even more surprising, they found that the C3dg component had anti-inflammatory properties on its own, a finding they are exploring in follow-up studies.
Collier explains the strategy succinctly. “We are using nanomaterials to induce the body’s immune system to become an anti-inflammatory antibody factory. It is essentially an anti-inflammatory vaccine.”
The team continues to study how their nanomedicines stimulate the immune system to optimize treatment and adapt the technique to treat other inflammatory diseases. They next plan to test the approach in a mouse model of rheumatoid arthritis.
“Over time, using this approach in patients would be a completely new way to treat inflammatory disease,” explains Collier. “Patients would require fewer doses than with current monoclonal antibody treatments and at lower cost, which is critical to reaching all patients, especially those in underserved settings and communities.”
The study appears in the Proceedings of the National Academy of Sciences (1).
This research was funded by Grant R01EB009701 from the National Institute of Biomedical Imaging and Bioengineering, the National Institute of General Medical Sciences, the National Science Foundation, the North Carolina Biotechnology Center, and Duke University.