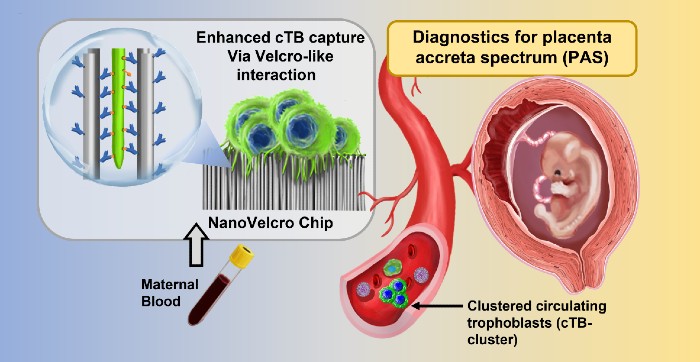
Placenta accreta spectrum disorder (PAS) is a life-threatening condition that occurs when the placenta remains attached to the uterus after childbirth. Now, NIBIB-funded researchers have developed a blood test for rapid identification of this condition, allowing timely intervention by specialists in high-risk pregnancies.
Although it occurs in less than 0.5% of pregnancies, PAS disorder is a serious condition in which the placenta becomes anchored too deeply in the uterine wall during pregnancy. The condition can cause significant blood loss during labor and delivery, requiring blood transfusions and intensive care to avoid serious complications or, more tragically, the death of the mother.
Currently, mothers with a history of pregnancy complications are examined with ultrasound for indications of PAS. However, women without such a history may also be at risk for PAS, making the current screening protocol insufficient.
Developed by an international team from UCLA and Cedars Sinai Medical Center in Los Angeles, the University of Utah in Salt Lake City, and research institutions in China, the blood test can be performed in the first trimester of pregnancy, allowing for early referrals to doctors specializing in high-risk pregnancies.
The new approach uses a technology called NanoVelcro Chip, which has been developed over the past 15 years by Dr. Yazhen Zhu and Hsian-Rong Tseng, professors of medical and molecular pharmacology at UCLA. The chip was originally created to detect tumor cells in the blood of cancer patients. The team adapted the chip so that it could detect placental cells in maternal blood samples.
The chip detects a specific cell type associated with the occurrence of PAS known as circulating trophoblasts. The NanoVelcro test is relatively simple and was designed to be easily performed within the normal workflow of healthcare facilities providing prenatal care. Only 2 milliliters of blood are needed, from which the cells are isolated and incubated on the test chip overnight. The number of trophoblasts and trophoblast clusters captured by the NanoVelcro chip is then counted using a fluorescent microscope. Therefore, the identification of an abnormal number of trophoblasts and trophoblast clusters (indicating an increased risk of PAS) can be determined in less than 24 hours.
“The development of this type of innovative yet simple test for PAS is an outstanding example of the types of technologies that the National Institute of Biomedical Imaging and Bioengineering (NIBIB) supports to improve maternal and prenatal care around the world,” explains Tiffani Lash, PhD, director of the NIBIB program in Point-of-Care Diagnostic Technologies.
In tests performed on more than 100 women, the blood test had an 83.8% probability of confirming the presence of placenta accreta and a 92% probability of ruling it out with a negative result.
“Recent population studies have shown that between half and two-thirds of cases of PAS disorders remain undiagnosed before delivery, highlighting the crucial need to develop new technologies for prenatal screening,” explains Dr. Margareta Pisarska of Cedars Sinai Medical Center, co-senior author of the work. “Our study demonstrates a promising non-invasive technology for detecting PAS that does not rely on expensive imaging instruments or expertise, making it accessible for a variety of point-of-care settings, including in low-resource areas.”
The team attributes the success of their work to a multidisciplinary approach that brought together experts in obstetrics, pathology, nanotechnology, engineering and microfluidics. The group is currently exploring ways to refine the test to improve its accuracy and reliability.
The study was published in Nature Communications. [1]
This work was supported by National Institutes of Health Grant U01EB026421 from the National Institute of Biomedical Imaging and Bioengineering, the National Cancer Institute, the National Institute of Child Health and Human Development, the National Center for Advancing Translational Science, and the Iris Cantor-UCLA Women’s Health Center Executive Advisory Board.