Prostheses can greatly improve an amputee’s quality of life, but current lower extremity devices cannot provide continuous neural control of balance or posture, which can lead to a variety of consequences, such as difficulty walking on certain surfaces or an increased likelihood of falling. Now, NIBIB-funded researchers are working on an ankle prosthesis that relies on the wearer’s residual muscles (and the electrical signals they generate) to help amputees with their postural control.
Approximately 2 million people living in the United States have had an amputation, and approximately 185,000 amputations are performed in this country each year.[1] Lower extremity amputations are the most common and many people will choose to use a prosthesis to assist with walking and other types of ambulation. Most lower limb prostheses are passive devices, which are designed to store and return energy during walking, but do not provide energy or allow a normal range of motion. Meanwhile, most powered prosthetics can help the user move the residual limb, but require external sensors to help anticipate the user’s movement. This prototype ankle prosthesis, on the other hand, is controlled by directly mapping the electrical activity generated by the user’s muscles without the need for external sensors or complex automation. The motorized and neurally controlled prosthetic device relies on training the user’s residual muscles to create continuous control of their posture and balance.
“This study is the culmination of work demonstrating that a prosthetic user can be trained to provide continuous neural control during various postural tasks,” said Grace Peng, Ph.D., NIBIB program director in Mathematical Modeling, Simulation and Analysis. “This case study is an important first step in implementing this unique control framework for future ‘user-driven’ prosthetic devices.”
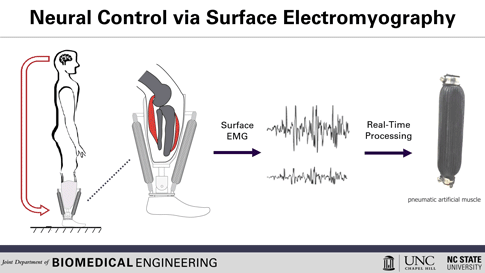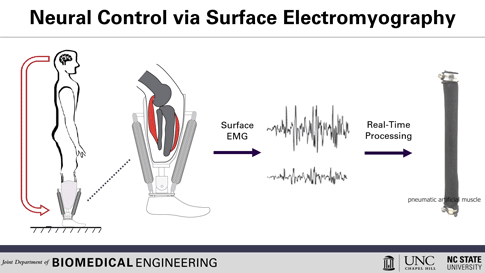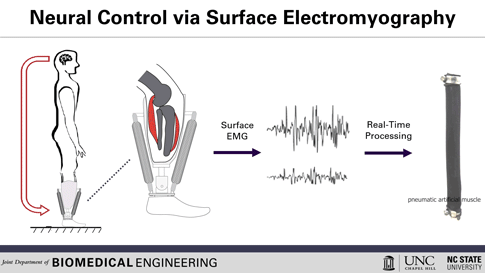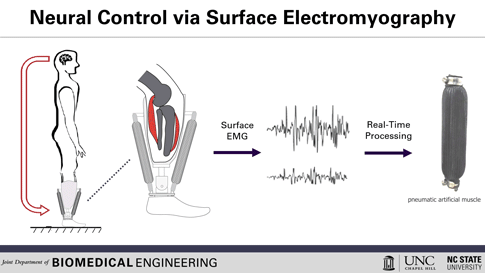
To use a joint, whether consciously or unconsciously, our brain sends an electrical signal to the necessary muscle groups, resulting in the contraction of the muscle fibers and allowing movement. After amputation, the muscles in the stump can still receive these electrical signals, explained Helen Huang, Ph.D., professor of biomedical engineering at North Carolina State University (NC) and the University of North Carolina (UNC) at Chapel Hill. His research group, hoping to harness that signaling ability, is working to develop a lower-extremity prosthetic device operated solely by the body’s own electrical signals, a concept known as direct electromyographic (or dEMG) control. Huang and his team recently reported in Wearable Technologies their case study evaluating this dEMG-controlled prosthesis, operated on by an individual with a transtibial amputation (where the limb is removed below the knee).[2]
This is how the prosthesis works: surface electrodes are placed on the residual limb, which can detect electrical signals in the user’s residual muscles. When the user contracts residual muscles, with the intention of flexing the foot, for example, the electrodes collect and process the associated EMG activity. This EMG signal drives pneumatic artificial muscles, which operate using pressurized air to contract or extend, allowing the user to continuously control the movement of the artificial limb.
“Often, if we have part of our limb removed, we start using the muscles in the stump a little differently,” Huang explained. This can result in altered muscle contraction patterns, and when a dEMG prosthetic device is used, the intended movement of the limb and the actual movement of the limb will not be synchronized, he said. For this reason, the prosthesis user, with the help of a physical therapist, “trains” his or her residual muscles to better operate the device. The training, which lasted three weeks in this case study, included activities of daily living that require postural control, such as transitioning from sitting to standing, lifting a heavy object, or reaching forward. “This regimen helps the user train their muscles (and brain) to better control the ankle prosthesis,” explained Aaron Fleming, a graduate student in Huang’s lab and first author of this case study.
After training, Huang and his colleagues assessed the participant’s stability while performing specific tasks, either with their usual passive ankle prosthesis or with the new dEMG-controlled device. His stability improved markedly when wearing the dEMG-controlled device, even when standing on a foam surface (which requires additional postural control) or with his eyes closed (which also challenges balance). The researchers also looked at the synchronization between his intact limb and his artificial limb and found that it was much greater when he was wearing the dEMG-controlled prosthesis compared to his usual passive device, both when standing and when lifting a heavy load.
“Postural stability is something that many healthy people take for granted,” Huang said. “Being able to stand in a crowded space without being afraid of falling if someone bumps into you is one of the many challenges people with lower extremity loss face,” he said. “While this research captures only one case study, it demonstrates the feasibility of developing a device that allows the user to intuitively adjust their posture, which could greatly increase their quality of life.”
Huang mentioned that they are currently investigating the use of their ankle prosthesis among several other participants with lower extremity loss, and that they will evaluate additional tasks beyond those in this case study, including walking.
This work was supported by grant EB024570 from the National Institute of Biomedical Imaging and Bioengineering (NIBIB); through grant F31HD101285 from the National Institute of Child Health and Human Development (NICHD); and by the National Science Foundation (NSF).
[1] Loss of lower limbs statistics provided by the Amputee Coalition.
[2] Aaron Fleming, Stephanie Huang, Elizabeth Buxton, Frank Hodges and Helen Huang. “Direct continuous electromyographic monitoring of a motorized ankle prosthesis to improve postural control after guided exercise training: a case study.” Wearable Technologies (2021), 2, e3. DOI:10.1017/wtc.2021.2