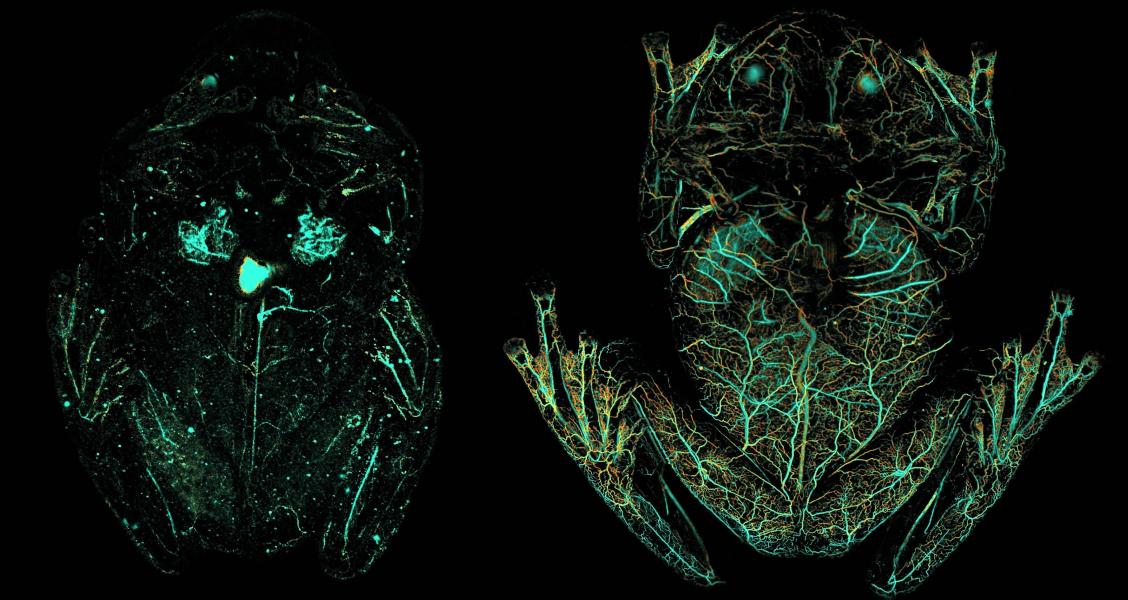
Tissue transparency, a useful ability for animals that want to hide from predators, is common in aquatic environments, but extremely rare on land. One exception is the glass frog, so called because its internal organs can be seen through its transparent skin and muscles. This frog is active at night and spends its days sleeping on leaves, becoming almost invisible in the foliage. But how exactly this creature becomes transparent has been a mystery.
Using cutting-edge imaging technology, NIH-funded researchers have found the secret behind the glass frog’s camouflage. His findings, recently published on the cover of Scienceshow that glass frogs can remove almost 90% of their red blood cells from circulation, storing them in the liver during rest. This mechanism, along with its inherently transparent tissues, allows the glass frog to increase its transparency two to three times, apparently as needed.
“In vertebrates like humans, tissue transparency is particularly difficult to achieve, as our circulatory systems are full of oxygen-carrying red blood cells that strongly absorb light and render our tissues opaque,” explained NIBIB-funded researcher Junjie Yao, Ph.D., assistant professor of biomedical engineering at Duke University. “In our study, we have discovered that the glass frog can hide almost all of its red blood cells within its liver on a daily basis, resulting in a unique form of camouflage that is distinct from all other known mechanisms of tissue transparency. Understanding this blood flow mechanism in the glass frog may provide insights into blood clotting disorders or strokes in humans.”
The glass frog’s camouflage is adaptive: the creature has maximum transparency when asleep and is at increased risk of being attacked. To understand the mechanism of this transparency, the researchers first used spectroscopy techniques to passively measure levels of Glassfrog hemoglobin (a protein in red blood cells that carries oxygen and absorbs light). They found that hemoglobin levels were barely distinguishable when the frogs were sleeping, but increased markedly after exercise. This means that the glass frog depletes its red blood cells from the circulation during rest, allowing for greater transparency and camouflage of the tissue during this vulnerable time.
But where are they exactly? Do these red blood cells disappear while the glass frog sleeps? To answer this question, the researchers used an advanced technique called photoacoustic microscopy, a hybrid imaging method that takes advantage of both light and sound waves. Here’s how it works: When a tissue absorbs light, some of that absorbed energy is converted into ultrasound waves. Measuring these ultrasound waves with a specialized transducer can provide detailed information about the molecules and tissues beneath the skin’s surface. In this study, the researchers optimized this technique to be able to detect the light absorbed by hemoglobin; In this way, the resulting ultrasound waves would provide information about the movement of the glass frogs’ red blood cells.
“Photoacoustic microscopy allowed us to capture the blood flow dynamics of glass frogs, even though those changes occur deep in their opaque internal organs,” explained Carlos Taboada, Ph.D., a postdoctoral associate at Duke University and one of the lead authors of this study. “This aspect of the technique was really important, because many internal organs of the glass frog contain millions of nanocrystals that attenuate most of the incident light. Traditional imaging methods cannot track blood flow with such a high level of precision or tell us exactly where glass frogs store their red blood cells.”
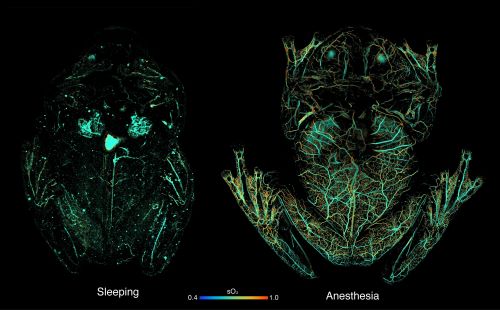
Using photoacoustic microscopy, the researchers imaged the glass frogs while they were under anesthesia (which causes red blood cells to distribute throughout the body), while they were sleeping, and after exercise. During the rest, the researchers found that the glass frogs’ circulating pool of red blood cells decreased by 80 to 90%, with the hemoglobin signal concentrated in the liver. Imaging of the frogs after exercise revealed that blood leaves the liver and returns to circulation during activity, and red blood cells reaggregate in the liver about an hour after movement.
By revealing the glass frog’s camouflage mechanism, a new question has arisen: How do these creatures concentrate most of their red blood cells in the liver without triggering clotting processes? “At this time, we really know very little about this important physiological function of glass frogs, but we are actively working to understand this phenomenon, which has important clinical implications,” Yao said. “This anticoagulant mechanism is highly relevant for treating thrombosis and stroke, as well as improving medical interventions that require removing blood from the body (such as dialysis), where blood clotting is a major concern.”
Future work in this space can take advantage of the unique ability of photoacoustic imaging to track glass frog blood flow, in a safe and unobtrusive way that does not disturb the animals. “By optimizing photoacoustic imaging to specifically detect hemoglobin, researchers were able to observe the natural blood flow dynamics of glass frogs without injecting contrast agents, representing a truly non-invasive approach,” said Randy King, Ph.D., program director in NIBIB’s Division of Applied Science and Technology. “What’s more, this study highlights how photoacoustic microscopy can be adapted to investigate different aspects of blood dynamics, providing high-resolution, real-time information.”
This study was funded by grants from NIBIB (R01EB028143), the National Institute of Neurological Disorders and Stroke (NINDS; R01NS111039), and the NIH BRAIN Initiative (RF1NS115581).
This Science Highlight describes a basic research finding. Basic research increases our understanding of human behavior and biology, which is critical to promoting new and better ways to prevent, diagnose, and treat diseases. Science is an unpredictable and incremental process: each research advance builds on past discoveries, often in unexpected ways. Most clinical advances would not be possible without knowledge of fundamental basic research.
Study reference: Carlos Taboada et al. Glass frogs hide blood in their liver to maintain transparency. Science (2022). DOI: 10.1126/science.abl6620