New research suggests that cells known to rebuild skin after injury, called fibroblasts, may do double duty, also clearing damaged tissue in burns and other wounds to make room for new skin.
Researchers at Boston University and Harvard University discovered this cellular ability by simulating cuts and burns in a miniature skin tissue model and monitoring how fibroblasts responded. The results of a study published in APL Bioengineering show that in charred samples, fibroblasts consumed and removed the decomposed tissue, a process that scientists previously thought was carried out only by immune cells. The study authors suggest that the finding is an important first step toward developing future therapies that accelerate and optimize burn healing.
“Our bodies need to remove dead tissue from wounds before they begin to repair. But if that phase takes too long, then the wound will not repair at all,” said Boston University professor Jeroen Eyckmans, lead author of the study. “You have to shorten that first period as much as possible.”
In the clinic, healthcare professionals already take a “out with the old, in with the new” approach, prioritizing cleaning burns. However, science is lacking on how the body responds differently to burns and other types of injuries at the cellular level, limiting therapeutic options, Eyckmans said.
To begin to unravel the healing process at a more fundamental level, Eyckmans and his colleagues previously designed a microtissue model composed of fibroblasts buried within a small sheet composed of collagen, one of the main components of the structure, or extracellular matrix (ECM), that supports the cells within the skin.
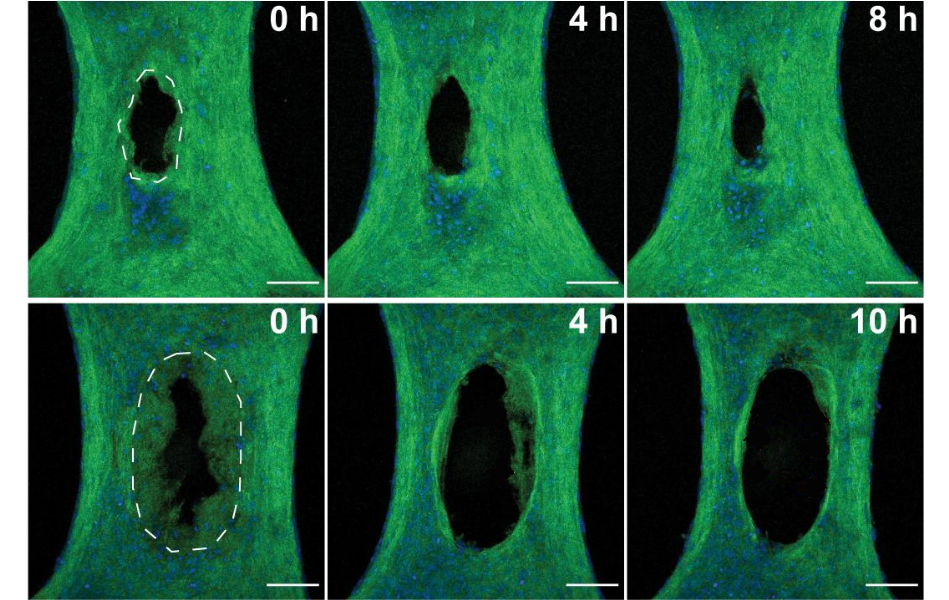
Initially, the researchers created wounds in the model exclusively with a half-millimeter-wide diamond disc, but the lacerations were so small that it was difficult to examine how the cells responded. In the new study, they also targeted tissues with lasers, simulating burn injuries that would be larger and easier to study.
The researchers compared how cells responded in both types of injuries by evaluating microscopic images collected in the hours after the injuries. With a clear view of the injured microtissue, they noted many similarities between the two types of wounds, but also several key differences.
While the knife damaged tissue immediately at the incision site, sparing the cells and ECM adjacent to the wound, the heat generated by the laser created a ring of damaged tissue around the site where the laser struck.
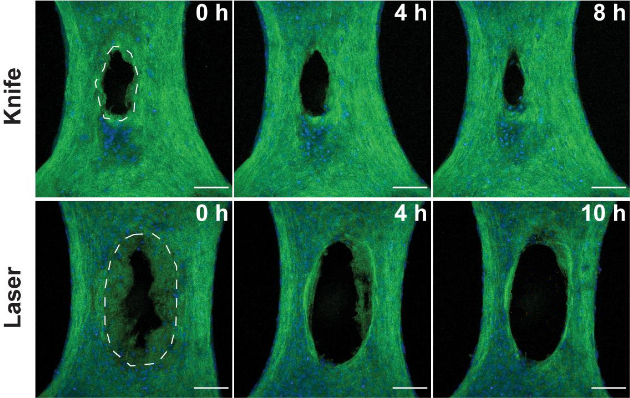
The knife wound began to close within minutes as expected, but the burn began to widen over the course of many hours. The researchers were surprised by the removal of damaged tissue in burns, as their model excluded any immune cells, which normally lead the removal of damaged tissue.
Close inspection revealed that fibroblasts were cleaning up the laser-scorched ECM. Additional experiments, in which the team inhibited specific proteins within the fibroblasts, suggested that the cells were ingesting ECM through a process called phagocytosis, which is commonly carried out by immune cells.
“To my knowledge, this is the first study to show that fibroblasts can exhibit phagocytosis in a wound healing context,” Eyckmans said. “We now have an additional cell type to target that could speed up wound clearance.”
Through a drug, genetic modification, or some other means, future clinical treatments could potentially induce fibroblasts to initiate or accelerate ECM clearance, facilitating burn healing during the critical initial phase.
The laser injury model allowed Eyckmans and his colleagues to take their first look at wound removal at the cellular level, but the team does not intend to stop there. They have their sights set on expanding their model to examine how other cell types, such as those in the vasculature, heal and heal wounds.
“Wound healing is an incredibly multifaceted and complicated process, but the insights gained from this injury model complete an important piece of the puzzle. The findings could open a path to new clinical treatments in the future,” said David Rampulla, Ph.D., director of the Science and Technology Discovery Division at the National Institute of Biomedical Imaging and Bioengineering (NIBIB).
This study was funded by a grant from the NIBIB (R21EB028491), as well as grants from the National Science Foundation (2036842) and the Office of the Director of the National Institutes of Health (S10OD028571).
This Science Highlight describes a basic research finding. Basic research increases our understanding of human behavior and biology, which is critical to promoting new and better ways to prevent, diagnose, and treat diseases. Science is an unpredictable and incremental process: each research advance builds on past discoveries, often in unexpected ways. Most clinical advances would not be possible without knowledge of fundamental basic research.
Study reference: Megan Griebel et al. Removal of fibroblasts from damaged tissue after laser ablation in engineered microtissues. APL Bioengineering (2023). DOI: 10.1063/5.0133478
About the artwork: The second image is licensed under the Creative Commons Attribution 4.0 International license and has been modified for clarity in several areas of the NIBIB website.